Abstract
Background
The aim of this review is to explore whether patients with autoimmune diseases (AIDs) were at high risk of infection during the COVID-19 epidemic and how severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic affected immune system.
Methods
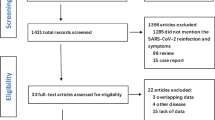
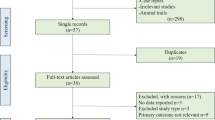
A systematic literature search was performed using the foreign databases (NCBI, web of science, EBSCO, ELSEVIER ScienceDirect) and Chinese databases (WanFang, CNKI (China National Knowledge Infrastructure), VIP, CBM) to locate all relevant publications (up to January 10, 2021). The search strategies used Medical Search Headings (MeSH) headings and keywords for “COVID-19” or “SARS-CoV-2” or “coronavirus” and “autoimmune disease”.
Results
This review evaluates the effect of SARS-CoV-2 on the immune system through ACE-2 receptor binding as the main pathway for cell attachment and invasion. It is speculated that SARS-COV-2 infection can activate lymphocytes and inflammatory response, which may play a role in the clinical onset of AIDs and also patients were treated with immunomodulatory drugs during COVID-19 outbreak. Preliminary studies suggested that the risk of developing severe forms of COVID-19 in patients with AIDs treated with immunomodulators or biologics might not increase. A large number of samples are needed for further verification, leading to an excessive immune response to external stimuli.
Conclusion
The relationship between autoimmune diseases and SARS-CoV-2 infection is complex. During the COVID-19 epidemic, individualized interventions for AIDs should be provided such as Internet-based service.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A novel coronaviruses (CoVs), called severe acute respiratory syndrome coronavirus-2 (SARS-CoV -2) or coronavirus disease 2019 (COVID-19), was first identified in December 2019 in Wuhan, Hubei Province, China [1]. Due to the relentless spread and pathogenicity of the virus, the World Health Organization (WHO) declared that COVID-19 outbreak have become a global public health concern on March 11th, 2020 [2]. To reduce the spread of deadly the disease and save lives, many countries have introduced emergency containment and control measures including isolate populations, wear a mask and hand sterilization [3]. To date, the number of confirmed reports of new infected persons and deaths is increasing every day across the world. Safe and effective vaccines against COVID-19 pandemic are urgently needed. Notably, COVID-19 vaccines buoy hope. On 11 December 2020, vaccine developed by Pfizer/BioNTech has been given approval for emergency use by the US Food and Drug Administration (FDA) [4]. On 18 December 2020, FDA issued an Emergency Use Authorization (EUA) for the Moderna’s mRNA-1273 vaccine which encodes the prefusion stabilized full-length spike protein of the SARS-CoV-2 [5]. It is necessary to screen contraindication and take precautions before administering COVID-19 vaccines to avoid serious adverse events.
Autoimmune diseases (AIDs) are characterized by generation of autoantibodies, which can activate the complement system and can mediate tissue damage and trigger systemic inflammation [6]. Currently, COVID-19 pandemic has raised concerns about patients affected with AIDs. There are two main reasons, one is immunosuppressants are used in the treatment AIDs which may lead to an increased susceptibility to COVID-19 [7]; the other is that the disease itself causes immune dysfunction. Presently, based on the knowledge of other viral infections and the effect of the therapies used in rheumatic patients has led physicians and patients to be concerned that patients with AIDs may be more susceptible to acquiring COVID-19 and may be at greater risk for adverse outcomes [8]. Recently, a comparative cohort study have demonstrated that systemic autoimmune rheumatic diseases patients had significantly higher risks of hospitalization, ICU admission, acute renal failure, and venous thromboembolism versus matched comparators but did not have significantly higher risks of mechanical ventilation or death [9]. Importantly, Caso et al. considered that the shared pathogenetic mechanisms and clinical–radiological aspects between hyperinflammatory diseases and COVID-19 suggest that SARS-CoV-2 may trigger the development of a rapid autoimmune and/or autoinflammatory dysregulation in genetic predisposed individuals, leading to severe interstitial pneumonia [10].
Evidence to date, however, has shown that AIDs patients do not seem to be at an increased risk of infection with SARS-CoV-2 compared with the general population, including in patients treated with immunosuppressant medications [11,12,13]. Additionally, previous studies have reported the possible antiviral activity of immune-modulating drugs against SARS-CoV-2 including hydroxychloroquine (HCQ), chloroquine (CQ) and tocilizumab—an anti-interleukin (IL)-6 receptor antibody, but there are still controversies [14, 15]. Notably, the symptoms of COVID-19 patients vary from asymptomatic to those with respiratory failure, complicated by ARDS, which may be in part due to an uncontrolled immune-response to SARS-CoV-2 infection triggering a systemic hyperinflammatory response, the so-called "cytokine storm" [16]. These cytokines, including IL-1, IL-6, tumor necrosis factor-α (TNF-α), are released, lead to increased vascular permeability, hemorrhage, and multi-organ failure [17]. More interestingly, one study hypothesized that in the context of SARS-CoV-2 infection, the cause of this transient lymphopenia may be several local inflammatory reaction and release of cytokines, which affect suppression of bone marrow, further, loss of tolerance to self-antigens and, ultimately, autoimmunity [18].
However, the impact of SARS-CoV-2 on AIDs patients remain fully unclear up to now. Thus, based on the available evidence, we will summarize the existing evidence related to the potential role of COVID-19 in AIDs.
Overview of COVID-19
Structure of SARS-CoV-2
Genomic structure of SARS-CoV-2 similar to that of typical CoVs mainly including an open reading frames (ORFs)1ab polyproteins at the 5′-terminal of the genome of SARS-CoV-2 and four major structural proteins:spike (S), envelope (E), nucleocapsid (N) and membrane (M) proteins. SARS-CoV-2 uses angiotensin-converting enzyme 2(ACE2) as host cell receptor to entry into cells [19].
Current status
As of 10 January 2020, 88,120,981 confirmed COVID-19 cases with 1,914,378 deaths have been reported according to WHO. COVID-19 cases continue to be reported globally from over 220 countries, areas or territories [2]. Notably, with no specific treatment available, as the statistics predict, the mortality and incidence rates of the COVID-19 are rising in global area on each successive day.
Identification
Shortly after COVID-19 occur, scientists determined that SARS-CoV-2 was responsible, which is a novel single-stranded RNA virus of the coronaviridae family. To avoid confusion with the recent strain SARS-CoV from 2002, the WHO called the new strain as SARS-COV-2 [2]. To date, by analyzing the whole genome of the pathogen SARS-CoV-2, researchers speculated that the coronavirus may have originated from wild animals (e.g. bat and pangolin), but the identified host is unknown [20]. Mean incubation period was around 6.4 days, ranges from 0 to 24 days [21]. Therefore, contacts were isolated under medical observation for 14 days.
Symptoms
The clinical manifestations of COVID-19 are diverse, but respiratory symptoms are often prominent and dry cough, fever and dyspnea are the most common. There can be progression to ARDS with pneumonia and pulmonary fibrosis secondary to a cytokine storm [22]. The elderly and patients with comorbidities such as hypertension and cardiac disease have been shown to be at higher risk [23]. Additionally, laboratory parameters showed COVID-19 patients have high levels of circulating multiple cytokine and/or anti-cytokine, suggesting cytokines may be prominent markers of the pathophysiology of SARS-CoV-2 infection [24].
Transmission
Respiratory droplets and close contact are the main route of transmission [1], thus, public measures such as social distancing and self-isolation can be taken to reduce transmission rates.
Diagnosis
Real-time reverse transcription polymerase chain reaction (rRT-PCR) was used to detect nucleic acid of SARS-CoV-2 in nasopharyngeal, oropharyngeal swab, or the lower respiratory tract samples [25]. Typical computed tomography (CT) findings also have the diagnostic value.
Treatment
A number of COVID-19 vaccine candidates have shown extremely promising results [26], which is expected to be used in the near future. Current treatment strategies of COVID-19 include symptomatic treatment and supportive care (oxygen, mechanical and circulatory support). Various antiviral and other drugs have been used in clinical treatment including HCQ, CQ, azithromycin (AZM), remdesivir, baricitinib and convalescent plasma (CP).
HCQ, CQ, azithromycin (AZM)
To examine risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. Recently, in a cohort study, the result have reported that patients with both AZM and HCQ had increased odds of death than patients with no HCQ or AZM [27]. Furthermore, meta-analysis found that the mortality difference was not significant, neither in HCQ treatment group nor in HCQ plus AZM treatment group in comparison to controls [28]. On the contrary, another retrospective multicenter cohort study have described that there were no significant differences in in-hospital mortality between patients who received HCQ with or without azithromycin and patients who received neither drug [29]. However, the interpretation of these findings may be limited by the observational design. Recently, randomized trials were conducted to explore the role of HCQ as a postexposure therapy for SARS-CoV-2 infection. Results were similar in the HCQ and usual-care groups with respect to the incidence of PCR-confirmed, symptomatic COVID-19. In other words, among persons with recent exposure excluded a clinically meaningful effect of HCQ as postexposure prophylaxis to prevent SARS-CoV-2 infection. In addition, HCQ also did not reduce the transmission of SARS-CoV-2 or the incidence of seropositivity, and a higher incidence of adverse events (with low severity) in HCQ group than in the control [30, 31]. Notably, a randomized controlled trial (RCT) and a retrospective study have suggested that HCQ did not shorten viral shedding in mild-to-moderate COVID-19 subjects [32]. In brief, HCQ should be used cautiously in the treatment of COVID-19 illness.
Remdesivir, baricitinib
WHO expert groups recommended mortality trials of repurposed antiviral drug—remdesivir in patients hospitalized with COVID-19. Unfortunately, remdesivir had little or no effect on hospitalized patients with COVID-19, as indicated by overall mortality, initiation of ventilation, and duration of hospital stay [33]. Additionally, three retrospective trials have found that the efficacy of baricitinib, with significant reduction of intensive care unit (ICU) admission, and deaths [34]. An observational, longitudinal trial have reported that baricitinib prevented the progression to a severe, extreme form of the COVID-19 disease by modulating the patients' immune landscape and that these changes were associated with a safer, more favorable clinical outcome for patients with COVID-19 pneumonia [35]. Drug combination therapy was also concerned. An observational cohort study have found that a combination of baricitinib with corticosteroids was associated with greater improvement in pulmonary function when compared with corticosteroids alone in moderate-to-severe SARS-CoV-2 pneumonia patients [36]. A double-blind, randomized, placebo-controlled trial evaluating baricitinib plus remdesivir in hospitalized adults with COVID-19. Combination treatment was superior to remdesivir alone in reducing recovery time and accelerating improvement in clinical status among patients with COVID, especially among those receiving high-flow oxygen or noninvasive ventilation. Serious adverse events were less frequent in the combination group [37]. Collectively, drug–drug interaction and safety concerns should be taken into account before the administration of the recommended drugs.
Convalescent plasma
Due to CP successfully used in other coronaviruses outbreaks and contains neutralizing antibodies against the virus, which may hold promise as treatment for COVID-19. Patients receiving CP transfusion significantly improved the clinical outcomes of COVID-19 patients with diabetes mellitus (DM), especially the cure rate and duration of hospitalization compared with that in COVID-19 patients with DM receiving conventional treatment [38]. Similarly, CP treatment was significantly associated with a higher rate of clinical improvement in moderate and severe cases of SARS-CoV-2 infection and also highlighted administration of CP was a safe treatment option for patients with COVID-19 illness, with a favorable outcome in the rate and time to clinical improvement [39]. However, there are still many issues to consider, such as the donor selection, indication, administration, and follow-up after transfusion should clearly defined in national guidelines; interval between COVID-19 symptoms or diagnosis and CP transfusion.
Outcomes
Case fatality rate (CFR) varies geographically. Pooled CFR among confirmed COVID-19 patients was 0.02 (95% CI 0.02, 0.03) [40] (see Fig. 1).
COVID-19 and immune functions
Up till the present moment, the most significant predictors of disease severity relate to either activation or suppression of the host immune response. From this perspective, we will discuss the role of both innate and adaptive immune responses in SARS-CoV-2 infection (see Fig. 2).
Innate immune responses and SARS-CoV-2 infection
Innate immunity is the first defense line to resist the invasion of SARS-CoV-2. When the new virus is able to escape innate immune responses, it can proliferate without hindrance in primarily infected tissues. Subsequently, the tissue associated damage caused by the virus could induce the exaggerated production of proinflammatory cytokines, which the recruitment of proinflammatory macrophages and granulocytes and the generation of immune complexes [41]. In addition, dysregulated production of proinflammatory mediators contributes to ARDS and cytokine storm syndrome, then leading to further tissue damage [42], which may be part of the reason for the relatively high CFR. Reduction in percentages of some innate immune cells may play an important role in COVID-19. For example, the potential association of low eosinophil count and poor prognostic marker in COVID-19 patients was reported [43]. Also, association of COVID-19 severity with proinflammatory cytokines and other immune cell subsets was investigated.
Natural killer
Natural Killer (NK) cells are innate immune responders vital for viral clearance and the control of viral infection [44]. Accumulating researches focused on the immune status of NK cells and the severity of disease in COVID-19 patients. Used single-cell RNA-sequencing on the cells extracted from bronchoalveolar immune cells in patients with COVID-19 (moderate and severe) and healthy controls (HC), Liao et al. reported that bronchoalveolar lavage fluid (BALF) of patients with COVID-19 infection contained significantly higher proportions of NK cells compared to HC, but the proportion of NK cells in patients with severe diseases was decreased than those with moderate infection COVID-19, and NKG2A and CD94 were highly expressed by NK cells [45]. Intriguingly, Demaria et al. suggested that a reduce in NK cell numbers (including absolute numbers and the proportion of mature NK cells) as well as a dysfunctional state of these cells in the blood and lungs of COVID-19 patients than HC, which indicates NK cells do not seem to be associated with the exaggerated inflammatory response observed in ARDS [46]. Similarly, reduced numbers of NK cells in the peripheral blood of COVID-19 patients, which is associated with severity of the disease and prognosis [47]. Meanwhile, severe COVID-19 patients had significantly lower numbers of circulating NK cells compared with those in mild disease cases and HC, as well as recovering from COVID-19 had higher numbers of NK cells expression than those with active disease [48, 49]. Importantly, further research showed that total number of NK cells was lower markedly and the function of NK cells was exhausted with the increased expression of NKG2A in COVID-19 patients with severe pulmonary inflammation [49]. Additionally, several studies have described that high levels of CD39, CD94, death-ligand 1(PD-L1), and NKG2A (all possibly inhibit the function of T and NK cells) expression were also observed in NK cells isolated from the BALF of ARDS COVID-19 patients [45, 46], suggesting therapies targeting these molecules may contribute to virus elimination by boosting NK cells antiviral immunity at the early stage of SARS-CoV-2 infection. Collectively, these results indicated that due to the loss of NK cells, non-specific immunity is suppressed to a certain extent during COVID-19 infection and it is necessary to early improve NK cells antiviral activity to inhibit the course of the disease progression.
Macrophages
Macrophages are an integral component of innate immunity, which are important for immune response upon injury or infection [50]. Recent researches has revealed to some extent, macrophages may contribute to the disease progression of COVID-19 patients. Park et al. described that ACE2-expressing macrophages contained SARS-CoV-2 nucleoprotein antigen and showed upregulation of IL-6, which may contribute to the excessive inflammation and correlate with disease severity [51]. In mice infected with SARS-CoV-2, the typical histopathology was interstitial pneumonia, especially, alveolar interstitium were filled with infiltration of significant macrophages and lymphocytes, and accumulation of macrophages in alveolar cavities [52]. Remarkably, the viral infection of aggregated alveolar macrophages was obvious from early phase to the late stage in COVID-19 case. In addition, several chemokine and inflammatory cytokines secreted by SARS-CoV-2-infected alveolar macrophages including IL-6, IL-10, TNF-α with specific antibodies and PD-L1 [53]. These observations implied alveolar macrophages might be pivotal in the pathological changes in COVID-19 patients and macrophages can be a key player of the so-called cytokine storm or macrophage activation syndrome(MAS) by affecting inflammatory monocytic cells, neutrophils and T cell though various chemokines and may be damaging to the tissues [45]. Potential mechanisms were explored the function of macrophages in response to SARS-CoV-2. It is likely that the spike protein (S) of SARS-CoV-2 invades macrophages by binding to ACE2 receptor on the surface of immune cells in lung tissue, thus inducing lung inflammation and injury, indicating the role of abnormally activated macrophages as host cells of SARS-CoV-2 in the pathogenesis of COVID-19 [54]. Besides, coronavirus spike protein induces innate immune response of human monocytes/macrophages by activating NF-κB pathway in vitro [55]. Interestingly, a study observed that few children die from COVID-19 compared to adults in Italy, and then discussed whether macrophages heterogeneity may be important in determining COVID-19 lethality [56]. It is speculated that macrophages populate the important organs including lung in three "developmental waves" and the lungs of children have more macrophages of the first two waves rather than those of the third wave. In other word, different responses to COVID-19 may be related to the number of macrophages in the lungs at the first contact. The molecular mechanisms underlying about different populations of macrophages in COVID-19 disease deserves further study.
Dendritic cells
Dendritic cells (DCs) are professional antigen presenting cells (APCs) that bridges both innate and adaptive immune response [57]. Also, they are potent producers of cytokines that may contribute to immunopathology [58]. Researches reported that associations of specific DC subsets with disease progression in COVID-19 patients. To evaluate the impact of SARS-CoV-2 infection in circulating DCs, using a tSNE tool, specific DCs subsets in the three groups of COVID-19 patients were examined by Sanchez-Cerrillo et al. The finding exhibited CD123hi plasmacytoid DCs (pDC) and CD141 + conventional (cDC) were significantly diminished in the three COVID-19 patient (mild, severe, critical) groups, suggesting a disease-rather than severity-related impact on these cells [59]. Recently, Yang et al. suggested that the SARS-CoV-2-infected DCs in the lungs of COVID-19 patients might be a source of proinflammatory cytokine production that exacerbates the clinical manifestation of COVID-19 [60]. Furthermore, the results showed that attenuated interferon and proinflammatory response to SARS-CoV-2 in human DCs was associated with viral antagonism of STAT1 phosphorylation [60]. In addition, the researchers noted that patients with the same critical condition had different outcomes. Wei et al. displayed that on admission, COVID-19 patients with good prognoses had significantly higher frequency of CD14+ CD11C+ HLA-DR+ subset of dendritic cells compared to those with poor prognoses, suggesting dysregulation of the immune response may affect the outcome of critical COVID-19 patients [61]. These studies increases the knowledge on the role of specific subsets of DCs to SARS-COV-2 infections.
Monocyte
Monocytes are vital effectors and regulators of immune response which stay in the blood for a short time and migrate to inflammatory tissues [62]. In patients with severe SARS-CoV-2 infection, monocytes may play an important role in the pathological process of extensive lung injury caused by cytokine storm. Sanchez-Cerrillo et al. studied the frequencies and activation profiles of monocytes present in the blood of COVID-19 patients with different clinical severity in comparison with healthy individuals and have claimed inflammatory transitional and non-classical monocytes preferentially migrate from blood to lungs in patients with severe COVID-19 [59]. In cohort study, for SARS-CoV-2 PCR-positive patients, low monocyte on admission was found to be associated with severe acute respiratory illness (SARI)/critical disease. COVID-19 patients had higher percentages of monocytes compared with healthy subjects [63]. Significantly reduced non-classical (NC) and intermediate (INT) monocytes were found in acute patients with severe SARS-CoV-2 symptoms, but increased in patients with moderate symptoms NC [64]. Unlike, severe COVID-19 cases had lower levels of monocyte than non-severe ones. More deeply, an observational study noticed that COVID-19 symptomatic patients possessed higher monocyte distribution width mean value than those with paucisymptomatic [65]. Furthermore, an overview of the association of markers in the routine blood test with the severity of COVID-19 have demonstrated that no difference in the monocyte count (weighted mean difference 0.01 × 109/l; 95% CI -0.01 to 0.03) between severe and nonsevere groups [66]. Intriguingly, a review described changes in monocyte function and phenotype that were characteristic of both aging and severe COVID-19, which may explain increased morbidity and mortality of SARS-CoV-2 infection in the elderly [67]. On the other hand, immunological data of children with COVID-19 infection were also reported. Xiong et al. found that children with symptomatic COVID-19 infection had significantly higher monocyte counts [68]. Moreover, the causes of different severity of COVID-19 infection between children and adults can be analyzed in future.
Neutrophil
Neutrophils are the most numerous immune cells. Prior study have showed viruses can also induce neutrophil extracellular traps (NETs) [69]. Aberrant NETs formation can trigger a cascade of inflammatory reactions that results in permanent organ damage to the pulmonary, cardiovascular, and renal systems [70]. More importantly, extensively link excessive NET formation to ARDS and microthrombosis. NETs, as a source of extracellular histones, likely lead to ARDS and sepsis and intravascular NETs have been reported to play a major role in initiating and accreting thrombosis in arteries and veins [71, 72]. We speculate that neutrophils form a special structure after necrosis or apoptosis, called NETs, play a central role in the immunopathology of COVID-19 illness. The dynamic changes of neutrophil counts of patients with COVID-19 and their correlation with the disease severity have been reported. Severe COVID-19 cases showed significant and sustained increases in neutrophil counts and neutrophil–lymphocyte ratio (NLR) was also higher than mild cases and the older COVID-19 individuals had more chronic diseases and significantly elevated levels of neutrophil compared with the younger patients [73, 74], but among pediatric patients with COVID-19, moderate cases were associated with a decrease in neutrophil levels than those who with mild clinical characteristics [75]. Additionally, several studies have described elevated NLR was associated with adverse the clinical characteristics and death of COVID-19 patients and identified as an independent risk factor for severe COVID-19 infection [75,76,77], which suggesting that increased NLR can serve as an early warning signal, risk stratification and a prognostic utility of severe COVID-19. Interestingly, Wang et al. found that neutrophil to CD4+ lymphocyte ratio (NCD4LR) had a better performance than neutrophil to lymphocyte ratio in predicting the virus negative conversion [78]. The possible mechanism of neutrophils in viral infections of SARS-CoV-2 was explored. According to published RNA-seq data sets of lung cells and BALF cells infected with SARS-CoV-2, the results have revealed that infected cells expressed neutrophil-attracting chemokines such as CXCL1, CXCL2, CXCL3 IL-8 and upregulation of neutrophil genes (e.g. MUC21, CXCL1) and chemokines, suggesting likely involvement of neutrophils in COVID-19 lungs [79]. Furthermore, neutrophilia was associated with the development of ARDS and progression from ARDS to death in patients with COVID-19 [22]. On the other hand, the role of NETs in COVID-19 was assessed. Elevated levels of serum NETs in many hospitalized patients with COVID-19 and three markers (cell-free DNA, MPO-DNA, and Cit-H3) commonly used to detect net in blood were measured, and found that all the markers were significantly increased as well as sera from individuals with COVID-19 triggered NET release from control neutrophils in vitro [80]. Barnes et al. have speculated that treatments targeting NETs may reduce the clinical severity and mortality of COVID-19 [81]. In conclusion, these findings highlight neutrophils may be a target for the immunopathologic complications of severely ill COVID-19 patients.
Mast cell
Mast cells (MC) are important sentinel cells for host defense against specific pathogens [82]. There is increasing evidence for the importance of excessive mast cell activation in patients with severe acute as well as chronic ("long haul") COVID-19. Afrin et al. have described that much of COVID-19′s hyperinflammation is concordant with manners of inflammation which MC activation can drive and drugs with activity against MCs or their mediators have preliminarily been found to be meaningful in COVID-19 patients. Furthermore, hyperinflammatory cytokine storms may be rooted in an atypical response to SARS-CoV-2 by the dysfunctional MCs of MC activation syndrome (MCAS, usually due to acquired MC clonality) rather than a normal response by normal MCs in many severely symptomatic COVID-19 patients [83]. The potential mechanism may be MC activated by SARS-CoV-2 release histamine which increases IL-1 levels causing cytokine storm and inflammatory reaction in COVID-19, which can amplify the inflammatory process in the lung infected with SARS-CoV-2 [84]. In addition, MCs also communicate with endothelial cells, fibroblasts and macrophages further stimulating release of proinflammatory, fibrotic, thrombogenic and vasoactive mediators [85].
Adaptive immune responses and SARS-CoV-2 infection
Adaptive immune response is mediated by B cells and T cells, which plays a key role against virus [86]. Increasing evidence in characterizing the immune responses, especially adaptive immune responses to COVID-19 infection.
T cell
Recently, T cells including CD4 T and CD8 T play a fundamental role in SARS-CoV-2 infection. Several reports found SARS-CoV-2 infection may affect primarily T lymphocytes and the numbers of both CD4 T and CD8 T cells in moderate and severe COVID-19 cases were markedly decreased [47, 87], in particular, a severe decrease in CD8+ T cells and significantly elevated CD4/CD8 ratio was observed in COVID-19 patients which suggests CD8 T cells may be relevant to the disease severity and mortality of COVID-19 [88]. Strikingly, the clinical manifestations of COVID-19 infection are more consistent with subacute rather than acute viral illness, and this subacute progression pattern increases immunosuppression due to T cell depletion and exhaustion, leading to persistent COVID-19 virus persistence and patient death [89]. The mechanisms of peripheral T cell loss in moderate-to-severe COVID-19 remain unclear. T cell numbers were negatively associated with serum IL-6, IL-10, and TNF-α concentration, and with patients in the disease convalescent period suggesting decreased proinflammatory cytokine levels and restored T cell counts [90]. Another possible reason is that T cell recruitment from peripheral blood to sites of infection. Many studies observed that there were extensive infiltration of CD4T and CD8T cells in the lungs of patients with COVID-19 [45, 91]. The induction of robust T cell immunity is likely essential for efficient SARS-CoV-2 control. Additionally, researchers have further studied the effect of T cells on structural protein and non-structural region of betacoronaviruses. Le et al. suggested that the presence of CD4 and CD8 T cells recognizing multiple regions of the nucleocapsid protein (NP) [92]. Ni et al. showed that for recovered COVID-19 patients, the neutralizing antibody titers significantly correlated with the numbers of NP-specific T cells, further, receptor binding domain of spike protein (S-RBD)-specific T cell production of interferon(IFN)-γ, which indicated that S-RBD induced broader T cell immune responses [93]. Notably, T cell responses to structural NP and non-structural (NSP-7 and NSP13 of ORF1) regions of SARS-CoV-2 in COVID-19 patients who recently recovered from the infection were studied. Surprisingly, SARS-CoV-2 T cells exhibited a different pattern of immunodominance, frequently targeting the ORF1-coded proteins NSP7 and 13 as well as the NP structural protein in unexposed donors; subsequently, they showed that the recovered SARS patients still had long-lasting memory T cells reactive to SARS-NP, which displayed significant cross-reactivity to SARS-CoV-2 NP [92]. Collectively, these studies provide a preliminary direction for T cells response to SARS-CoV-2 infection, and conclusions need further confirmation.
B cell
B cells are important for the production of antibodies and protective immunity during COVID-19 infection. Recently, epidemiological studies have focused on the expression of B cells in patients with virus infection. Lymphocyte subsets count including B cell (CD19 +) were significant reduction in patients with severe/critical COVID-19 disease compared to mild/moderate disease [94]. A significant decrease of B cell is positively correlated with in-hospital death and severity of illness [95]. Measure/monitor the response of B cells to understand how the immune system responds to this harmful virus. Schultheiß et al. explored that the B cell response showed converging Ig heavy chain variable region 3 (IGHV3)-driven B cell receptor (BCR) clusters closely associated with SARS-CoV-2 antibodies [96]. SARS-CoV-2 elicits a robust B cell response, as evidenced by anti-RBD neutralizing IgG antibodies (nAbs) were detected in most infected patients [97]. Studies have increasingly explored a potential link between SARS-CoV-2 and antibody response. Antibodies can bind the SARS-CoV-2 protein including internal protein N and external glycoprotein S [98]. Strikingly, antibodies bind to the receptor binding domain of S protein with high immunogenicity, which can potentially neutralize and block the interaction between virus and host entering ACE2 receptor [97]. It may provide a new insight into clinical treatment and vaccine development. More importantly, after SARS-CoV-2 infection, the strength and durability of the immunity acquired by the human body has always been of great concern. Seow et al. described that at post onset of symptoms, antibody levels that inhibit the virus reach a peak after three weeks and then drop rapidly, and importantly, antibody immunization against the SARS-CoV-2 in convalescent patients may last only a few months [99]. Variously, S-specific antibodies, memory B cells and circulating follicular helper T cells were found in the plasma of convalescent patients with COVID-19, but the median value of blocking the interaction between ACE2 and virus binding site was weak (about 14%) in immune plasma of patients [100]. These results indicated that the role of immune system and SARS-CoV-2 infection in human body is complex, which needs to be further verified by large samples.
SARS-CoV-2 and autoimmune diseases
A better understanding of the implications of COVID-19 in AIDs patients is urgently needed to guide clinicians in the caring for patients with rheumatoid arthritis (RA), systemic lupus erythematous (SLE), multiple sclerosis (MS), inflammatory bowel disease (IBD), type 1 diabetes (TID), systemic sclerosis (SSc), ankylosing spondylitis (AS) and psoriasis (See Table 1).
Rheumatoid arthritis
RA, a chronic systemic autoimmune disease, is characterized by synovial joint inflammation [101]. There is great interest in understanding the outcomes of COVID-19 in RA patients, or whether it makes them more prone to rapidly progress into severe COVID-19. Schett et al. analyzed that the pattern of proinflammatory cytokines induced in COVID-19 has similarities to those targeted in the treatment of rheumatoid arthritis, but preliminary data from high-risk areas suggested that there is no an increased risk of COVID-19 in patients with inflammatory arthritis [102]. Previous study suggested that ambient respiratory viral infections including coronavirus in the population were associated with an increased number of incident RA. Similarly, a nationwide retrospective case–control study found that RA [odds ratio range (ORR), 1.207–1.244] showed significant association with risk of infection of COVID-19 [103]. A potential link between COVID-19 infections and the development of RA needs to be studied further. Interestingly, during COVID-19 epidemic, the implementation of a telemedicine programme for patients with RA in a few countries to reduce cross infection [104]. Rheumatoid Arthritis Impact of Disease (RAID) score could help identify the RA patients who are best suited to a telemedicine consultation as well as follow-up, and could also promote holistic management of subjective symptoms that might otherwise be overlooked [105]. However, whether telemedicine can effectively control the disease activity of RA and provide the best treatment is still questionable [106, 107]. In our opinion, first, it is important to assess the patient's willingness to receive a telemedicine. Second, several digital applications for monitoring disease activity should use and the risk of infection can be classified according to the treatment plan, age and complications of RA patients to make telemedicine even more reliable and improve efficiency and quality of healthcare assistance.
Systemic lupus erythematosus
SLE is an autoimmune disease with the production of autoantibodies against self-antigens and loss of immunological tolerance which causes tissue and organ damage [108]. Since the COVID-19 pandemic, whether SLE patients have a high risk of serious diseases has been widely concerned. Several studies speculated that SLE infection does not seem to increase the risk of SARS-CoV-2 [109, 110]. Also, Pablos et al. found that patients with SLE did not significantly increase the prevalence of PCR+ COVID-19 in hospitals [111], while Fernandez-Ruiz et al. have found that SLE patients with confirmed COVID-19 have a high rate of hospitalization but a similar mortality rate to the general population [112]. For SLE patients under immunosuppressive treatment, Gendebien et al. showed that there was not significant a higher rate of COVID-19 infection or symptoms [113]. Whereas, Wallace et al. have inferred that SLE patients may develop more severe manifestations of COVID-19 infection, even relative to patients with other AIDs [114], which may be due to the higher rate of black race, respiratory comorbidities, glucocorticoids and tobacco exposure in SLE patients. Noteworthy, from a novel perspective, a study found that epigenetic dysregulation could lead to an increased risk and severity of SARS-CoV-2 infection in patients with lupus, regardless of the concomitant immunosuppressive medications. Moreover, in lupus patients, ACE2 gene is demethylated and overexpressed, which encodes the receptor for SARS-CoV-2 spike glycoprotein, facilitating viral entry and enhancing viraemia [115]. Additionally, whether patients with SLE who have been treated with CQ, HCQ or others are infected with COVID-19. Konig et al. concluded that patients with SLE on baseline therapy with HCQ are not universally protected from COVID-19 [116]. Similarly, preliminary findings suggested that although most of the SLE patients received long-term treatment with HCQ and blood concentrations of the drug within therapeutic range, HCQ did not seem to prevent COVID-19, at least its severe forms, in patients with SLE [117]. Given interfering with coronavirus replication in vitro, the increased bioavailability of type 1 IFN in SLE possibly promotes host defenses against COVID-19 infection and adjunct therapy [118]. In summary, on the premise of taking measures to prevent SARS-CoV-2 infection, SLE patients should continue to receive reasonable treatment and many susceptible factors and comorbidities should be taken into account, which may prevent the serious complications related to COVID-19. A larger cohort of SLE patients, infected with SARS-CoV-2 is required to better understand the impact of COVID-19 on SLE patients and evaluate the clinical efficacy of these drugs (both antiviral and immunomodulatory).
Multiple sclerosis
MS is a chronic inflammatory, demyelinating neurodegenerative disease of the central nervous system (CNS) [119]. With the continuous spread of COVID-19, it is very important to evaluate the relationship between MS and COVID-19 and take appropriate protective measures in time. Castillo et al. have reported that COVID-19 is twice as common in people with MS as in the general population (OR 2.67; IC 95% 0.37–19.07), but there was not statistically significant [120]. Additionally, Louapre et al. have described Expanded Disability Severity Scale score (EDSS) ≥ 6 (OR 6.3, 95%CI 2.8–14.4) was independent risk factors for a COVID-19 severity score of 3 or more (indicating hospitalization or higher severity) [121]. Results from registry-based cohort showed that patients with MS was independent risk factors for severe COVID-19, while no association found between disease-modifying therapies (DMTs) exposure and COVID-19 severity [121]. Likewise, derived from a small sample size, the frequency measure of COVID-19 in patients with MS living in the Veneto region seems to be 2.5 times higher than general population, but DMT exposure did not seem to be associated with a high risk of COVID-19 or its complications, even in some cases of immunosuppression [122]. Furthermore, in the largest cohort study, death from COVID-19 was strongly associated with chronic neurological conditions including MS [123]. Interestingly, according to the formula, Cristiano et al. analyzed that these estimates would represent that every 21,163 cases of COVID-19, there could be expected one patient with MS infected in Argentina [124]. However, Fan et al. reported that there was no increased risk of COVID-19 infection was observed in patients with MS or neuromyelitis optica spectrum disorders (NMOSD) [125]. The possible reason is that a variety of stringent measures that have been taken to protect MS patients against risk of COVID-19 infection. The impact of immunosuppressive drugs used in the treatment of MS on COVID-19 severity was also explored. According to Italian Society of Neurology (ISN) and the Association of British Neurologists (ABN), both consider safe to start or continue treatment with first line (e.g. IFN-β, teriflunomide) and non-lymphodepleting second line DMTs such as fingolimod and natalizumab. For example, a case of COVID-19 in a patient with MS treated with natalizumab, with excellent recovery and repeated negative results in five consecutive microbiological studies, which may be due to the blockade of integrins induced by natalizumab [126]. It was a reasonable speculation that immune reconstitution induced by treatment may induce positive changes in the immune system in the defense against COVID-19. As for lymphodepleting DMTs (alemtuzumab, ocrelizumab, rituximab or cladribine), individual factors such as disease activity and lymphocyte count should be taken into account, but a temporary delay in initiation or administration is generally recommended (between 6 and 12 months) [127]. For instance, anti-CD20 monoclonal including ocrelizumab and rituximab antibodies increase the susceptibility of MS patients to SARS-CoV-2 infection[128], while several researches proposed that anti-CD20 monoclonal antibodies may have protective role against COVID-19 disease [129, 130]. Notably, these data were considered only preliminary, the effect of COVID-19 on MS should be interpreted cautiously. Further work is needed to provide the rationale for strategies regarding clinical management of patients with MS during the COVID-19 pandemic.
Inflammatory bowel disease
IBD, including ulcerative colitis (UC) and Crohn's disease (CD), are characterized by progressive and relapsing dysfunction of the gastrointestinal tract [131]. As the COVID-19 rapidly spread, both physicians and patients have been deeply concerned about the possible increased risk of COVID-19 infection in patients with IBD. In a study of 9177 IBD patients in USA, 32 were reported to have confirmed COVID-19 (0.3%, 95% CI 0.1–0.5%) [132]. Despite a few studies found that patients with IBD more likely to be affected by the COVID-19 pandemic [133, 134]. Fortunately, overall available evidence suggests that IBD patients, even patients treated with medication, might have no increased risk of developing COVID-19 [135, 136], suggesting medical therapy with immunomodulators or biologics may not increases the risk of this infection. Also, information regarding the characteristics and prognosis of SARS-CoV-2 infection in patients with IBD was required. Patients with IBD and a positive test for SARS-CoV-2 have a good overall prognosis and severe outcomes of COVID-19 were rare and comparable to similarly aged individuals in the general population [137, 138]. New-onset diarrhea and abdominal pain were significantly more frequent in IBD patients with COVID-19 than without, which highlights the need for COVID-19 evaluation in IBD patients with new gastrointestinal symptoms [139]. More importantly, factors determining susceptibility to COVID-19 in IBD patients were identified, Krzysztof et al. analyzed that age, smoking, the presence of inflammation and anatomical location were key determinants which effect expression of virus receptor ACE2 and TMPRSS2 in IBD patients [140]. In addition, SARS-CoV-2 was detected in fecal samples from patients with COVID-19, and high concentrations of ACE2 were found in the inflamed intestinal ileum and colon of IBD patients. The results may provide a possible mechanistic link. Based on the emerging evidence and high risk factors, such as age, disease activity, drug therapies and comorbidities were also considered, many expert have proposed that specific guidelines and recommendations (such as use of immunomodulatory drugs and protection of daily life) for IBD patients including adults and children in the current pandemic era to assure the safety of ongoing treatment and adjust individualized care [141,142,143]. It should be noted that the adoption of these recommendations should take into account the occurrence of local COVID-19 and combine with local guidelines and isolation measures to seek the optimal solution. During the epidemic period of the SARS-CoV-2, to improve the communication between doctors and patients, and to provide specific suggestions for IBD patients, some alternative solutions have been implemented, including restrictions on infusion devices, telemedicine, online drug delivery services and patient education on measures to prevent infection [144].
Other autoimmune diseases
TID, also known as insulin-dependent diabetes mellitus, an autoimmune disorder, is usually diagnosed with insulin deficiency [145]. Data regarding potential effects of SARS-CoV-2 infection on patients with T1D are emerging. Vangoitsenhoven et al. have showed that community-dwelling people with T1D did not have an increased risk of worse COVID-19 outcomes defined as hospitalization for COVID-19 or mortality [146]. To evaluate the impact of lockdown on glucose control in T1D patients. Capaldo et al. found that adults with T1D had improved glucose control indicated by increased time in target range, reduced glucose variability, and reduced hyperglycemia and severe hypoglycemia, indicating the importance of a more stable rhythm of life, including more regular mealtimes [147]. Further, using a hybrid closed loop (HCL) system, Tornese et al. showed that the glycemic control of T1D in adolescents did not worsen during the restrictions due to COVID-19 pandemics and further improved in those who continued physical activity (PA) during the quarantine [148]. Patient characteristics and adverse outcomes among patients with T1D diabetes with confirmed COVID-19 was also explored. Patients were classified as COVID-19-positive case group and COVID-19-like case group, the most prevalent adverse outcome was diabetic ketoacidosis (DKA) and high blood glucose in both groups [149].
SSc is an autoimmune disease characterised by fibrosis of the skin and internal organs and vasculopathy [150]. Because interstitial lung disease (ILD) is a common complication seen in patients with SSc, there has been concern that patients with SSc may be at increased risk for acquiring COVID-19 and/or at increased risk for adverse outcomes. To date, however, this has not been demonstrated. The World Scleroderma Foundation have provided answers to the main practical questions for physicians and SSc patients (Up to date 14 April 2020) and Minniti et al. have proposed preliminary advice on how to take care of these patients during the COVID-19 pandemic [151, 152]. For example, patients should continue immunosuppression to avoid SSc relapses and stopping or reducing therapy should be agreed with physician. When family members or themselves are infected, immunosuppressive drug interruption may be advised. There is no evidence to support that SSc patients benefit from additional supportive/preventive therapy including prophylactic use of drugs and adjunctive therapy. Telemedicine consultations and assessments are encouraged. For severe SSc-COVID-19-infected patients, antiviral therapy or tocilizumab may be a rescue treatment to avoid ARDS. The routinely use of corticosteroids for treatment of viral pneumonia is not recommended and should be carefully employed in SSc. Drug-induced immunosuppression and risk of SARS-CoV-2 infection were explored in SSc patients. Patients with systemic sclerosis treated with rituximab might not exhibit an increased risk of severe COVID-19 and a careful follow-up is required, because these patients may experience a delayed progression [153].
AS is a chronic inflammatory disease, characterized by the enthesitis of sacroiliac joints and the spine [154]. To date, little is known with regard to the effect of COVID-19 among patients with AS. A 61-year-old patient with AS who presented with gastrointestinal symptoms, newly onset fever and respiratory manifestations following the admission, then, RT-PCR test for SARS-CoV-2 resulted positive. Substantial clinical improvement was achieved with HCQ, prednisolone, tocilizumab and enoxaparin sodium [155]. Another case reported that a COVID-19 patient with AS treated with etanercept had olfactory and gustatory sensory dysfunction [156]. More studies are warranted to analyze clinical characteristics and outcome of COVID-19 patients with AS on immunosuppressive agents.
Psoriasis is a common chronic and recurrent inflammatory skin disease [157]. The prevalence of COVID-19 in psoriasis/autoimmune skin diseases patients was 0.011 (95% CI 0.006–0.021), and the mortality due to COVID-19 in patients with psoriasis/autoimmune skin diseases was 0.097 (95% CI 0.042–0.21) [158]. A single-center case–control study have suggested that psoriasis patients on biologics displayed higher risk to be infected and to be hospitalized/self-quarantined at home, but ICU hospitalization and death did not differ from the general population. Besides, comorbidities associated with psoriasis (such as hypertension, obesity, and diabetes) worsen the outcome of COVID-19 illness [159]. It is worth exploring whether immunosuppression or immunoregulation makes psoriasis patients receiving biotherapy more susceptible to COVID-19 infection. Using randomized clinical trials (compared overall infection rates, rates of upper respiratory infections and nasopharyngitis), Lebwohl et al. have found that the rates of nasopharyngitis and upper respiratory tract infections are similar to placebo [160]. Similarly, compared to the general population, there is no increased susceptibility for SARS-CoV-2 infection or increased severity of the disease course of COVID-19 in patients with psoriasis receiving systemic treatments, including biologics [161]. These studies suggested that biological therapy should be continued in patients with immune-mediated skin diseases during the COVID-19 outbreak. More importantly, dermatologists should provide best advice on the treatment of cutaneous immune-mediated diseases during the COVID-19 pandemic on an individual basis.
Effects of treatment for AIDs on SARS-CoV-2 infection
Understanding the impact of immunosuppressive therapy on the progression of COVID-19 in patients with AIDs can guide the physician to achieve an effective protective strategy or, when infected, to optimise a real-time treatment. Patients were divided into two groups based on whether they are taking or not taking biologics (e.g. TNF inhibitors, IL-6 inhibitors, kinase inhibitors, etc), the results have showed that the group on immunosuppressive drugs had significantly lower odds of hospitalizations and ICU admissions compared with the other group, but the risk of COVID-19 illness remains the same between the study groups [162]. Similarly, COVID-19 is more frequent in the subgroup of autoimmune systemic diseases patients without ongoing conventional synthetic disease- modifying anti-rheumatic drugs (HCQ and methotrexate) [163]. Case series of individuals with 600 rheumatic disease patients from 40 countries have demonstrated that biologic therapies and non-steroidal anti-inflammatory drug (NSAID) were not associated with a higher risk of hospitalisation for COVID-19, but anti-TNF with a decreased odds of hospitalisation in patients with rheumatic disease [164]. Interestingly, some hypotheses may explain why a higher incidence of fatal COVID-19 outcomes was not found in AIDs patients on systemic treatments. Patients with AIDs under systemic treatment may pay more attention to personal protection (e.g., hand disinfect, wearing a facial mask, social distancing) compared to the general population. Another hypothesis is that a possible protective effect against harmful manifestations of COVID-19 provided by biologics. Inhibition of the COVID-19 immune response would be harmful in the early phase of infection, but it would be helpful in the progression to the severe form of the disease [163]. Additionally, previous studies of coronavirus have shown adverse reactions including delayed virus clearance and potential complications in patients receiving high doses of corticosteroids [165, 166], thus, in the absence of controlled clinical trials on the use of corticosteroids, on September 2, guidance from WHO advises against the use of corticosteroids patients with nonsevere cases, but systemic corticosteroids were recommended for the treatment of severe and critical patients with COVID-19 [167]. Huang et al. have found that patients with corticosteroids use have significantly worse clinical outcomes including ARDS, shock, kidney injury, continuous renal replacement, secondary infection than patients who did not receive corticosteroids [1]. Meta-analyses of observational studies have concluded that glucocorticoid use increases the risk of SARS-CoV-2 infection and might contribute to the higher prevalence of COVID-19 in ADs [158]. Should the immunosuppressive treatment be stopped in AIDs patients during the pandemic? On balance, it is advisable that AIDs patients continue treatment [168, 169]. A reasonable explanation is that discontinuation of the use of biological increase the risk of flares of the underlying autoimmune condition, which itself is an increased risk factor for infections [170]. Notably, the treatment plan should comprehensively take into account the characteristics of individual patients, such as age, gender, complications and disease condition [159]. AIDs patients on biologics should be carefully monitored with telemedicine during COVID-19 outbreak and early treated. Several studies have evaluated the impact of anti-CD20 agents on immune response to vaccines. Humoral vaccine responses were significantly impaired by B cell depleting anti-CD20 monoclonal antibody therapies, particularly to a neoantigen [171, 172]. From published rituximab and unpublished ocrelizumab (NCT00676715, NCT02545868) trial data, protective neutralizing antibody and vaccination responses are predicted to be blunted in CD-20 treated people until naïve B cell repopulate, based on B-cell repopulation-kinetics and vaccination responses [173]. Due to vaccinations against SARS-COV-2 will begin in the near future, for patients already on anti-CD20 therapy, this may not make sense, since the immunosuppressive effects of these agents are long-lasting (usually greater than 6 months), but for patients currently considering anti-CD20 treatment, especially those whose overall survival is not improved by anti-CD20 treatment, physicians should carefully weigh the risk/benefit ratio of anti-CD20 treatment [172]. Additional longitudinal studies are needed to confirm these findings.
Conclusion and future challenges
Understanding the risk of acquiring COVID-19 in patients with AIDs remains critical, as does decision-making regarding management of immune modulatory therapy in this patient population during the pandemic. The relationship between autoimmune diseases and SARS-CoV-2 infection is complex. We have explored that the effect of SARS-CoV-2 on the immune system through ACE-2 receptor binding as the main pathway for cell attachment and invasion, also known as cytokine storm, which is characterized by hyper-activation of T cells and massive production of several cytokines. SARS-COV-2 infection can activate lymphocytes and inflammatory response, which may play a role in the clinical onset of AIDs disease and patients treated with immunomodulatory drugs during COVID-19 outbreak. Thus, it may be a reasonable speculation this pathogen may be associated with either the triggering or the exacerbation of AIDs disease. However, based on the current literature, preliminary data suggested that the real risk of developing severe forms of COVID-19 in patients with AIDs treated with immunomodulators or biologics might not increase. Notably, some experts have given recommendations and guidelines based on risk stratification and local epidemic prevention measures to guide clinicians in the care of patients. During the COVID-19 epidemic, the new management strategy for AIDs patients was used such as internet-based service to provide an individualized approach of AIDs interventions.
There are still several questions that remain unknown. For instance, the potential infectious risk of stool samples and human intestinal tract may serve as an alternative infection route for SARS-CoV-2. Moreover, does the presence of some clinical symptoms of AIDs, such as intestinal inflammation and fibrosis, can affect the clinical course of COVID-19? Is there a vicious circle between autoimmune diseases and COVID-19? The outcome of SARS-CoV-2 reinfection in autoimmune diseases is unclear. To date, there are no established specific therapies for this novel disease. We hope this work may contribute in a significant way to the understanding of the association between patient with AIDs and COVID-19 infection.
References
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet. 2019;395(10223):497–506. https://doi.org/10.1016/S0140-6736(20) (published correction appears in Lancet. 2020 Jan 30).
World Health Organization. Coronavirus disease (COVID-19) pandemic. who.int/ emergencies/diseases/novel-coronavirus-2019.
Flahault A. COVID-19 cacophony: is there any orchestra conductor? Lancet. 2020;395(10229):1037. https://doi.org/10.1016/S0140-6736(20)30491-8.
Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, et al. The advisory committee on immunization practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine: united states, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69(50):1922–4.
Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, et al. The advisory committee on immunization practices’ interim recommendation for use of moderna COVID-19 Vaccine: united states, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69(5152):1653–6. https://doi.org/10.15585/mmwr.mm695152e1.
Karagianni P, Tzioufas AG. Epigenetic perspectives on systemic autoimmune disease. J Autoimmun. 2019;104:102315. https://doi.org/10.1016/j.jaut.2019.102315.
Favalli EG, Ingegnoli F, De Lucia O, Cincinelli G, Cimaz R, Caporali R. COVID-19 infection and rheumatoid arthritis: Faraway, so close! Autoimmun Rev. 2020;19(5):102523. https://doi.org/10.1016/j.autrev.2020.102523.
Figueroa-Parra G, Aguirre-Garcia GM, Gamboa-Alonso CM, Camacho-Ortiz A, Galarza- Delgado DA. Are my patients with rheumatic diseases at higher risk of COVID-19? Ann Rheum Dis. 2020;79(6):839–40. https://doi.org/10.1136/annrheumdis-2020-217322.
D’Silva KM, Jorge A, Cohen A, McCormick N, Zhang Y, Wallace ZS, et al. COVID-19 outcomes in patients with systemic autoimmune rheumatic diseases (SARDs) compared to the general population: a us multi-center comparative cohort study. Arthritis Rheumatol. 2020. https://doi.org/10.1002/art.41619.10.1002/art.41619.
Caso F, Costa L, Ruscitti P, Navarini L, Del Puente A, Giacomelli R, et al. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19(5):102524. https://doi.org/10.1016/j.autrev.2020.102524.
Emmi G, Bettiol A, Mattioli I, Silvestri E, Di Scala G, Urban ML, et al. SARS-CoV-2 infection among patients with systemic autoimmune diseases. Autoimmun Rev. 2020;19(7):102575. https://doi.org/10.1016/j.autrev.2020.102575.
Zen M, Fuzzi E, Astorri D, Saccon F, Padoan R, Ienna L, et al. SARS-CoV-2 infection in patients with autoimmune rheumatic diseases in northeast Italy: a cross-sectional study on 916 patients. J Autoimmun. 2020;112:102502. https://doi.org/10.1016/j.jaut.2020.102502.
Favalli EG, Monti S, Ingegnoli F, Balduzzi S, Caporali R, Montecucco C. Incidence of COVID-19 in patients with rheumatic diseases treated with targeted immunosuppressive drugs: what can we learn from observational data? Arthritis Rheumatol. 2020. https://doi.org/10.1002/art.41388.
Yazdany J, Kim AHJ. Use of hydroxychloroquine and chloroquine during the COVID-19 pandemic: what every clinician should know. Ann Intern Med. 2020;172(11):754–5. https://doi.org/10.7326/M20-1334.
Misra DP, Agarwal V, Gasparyan AY, Zimba O. Rheumatologists’ perspective on coronavirus disease 19 (COVID-19) and potential therapeutic targets. Clin Rheumatol. 2020;39(7):2055–62. https://doi.org/10.1007/s10067-020-05073-9.
Alijotas-Reig J, Esteve-Valverde E, Belizna C, Selva-O’Callaghan A, Pardos-Gea J, Quintana A, et al. Immunomodulatory therapy for the management of severe COVID-19 Beyond the anti-viral therapy: a comprehensive review. Autoimmun Rev. 2020;19(7):102569. https://doi.org/10.1016/j.autrev.2020 (102569).
Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38(1):1–9. https://doi.org/10.12932/AP-200220-0772.
Cañas CA. The triggering of post-COVID-19 autoimmunity phenomena could be associated with both transient immunosuppression and an inappropriate form of immune reconstitution in susceptible individuals. Med Hypotheses. 2020;145:110345. https://doi.org/10.1016/j.mehy.2020.
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS- CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80. https://doi.org/10.1016/j.cell.2020.02.052.
Zhou P, Yang X, Wang X, Hu B, Zhang L, Zhang W, et al. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. Nature. 2020;579:270–3. https://doi.org/10.1038/s41586-020-2012-7.
Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020;92(6):568–76. https://doi.org/10.1002/jmv.25748.
Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan. China JAMA Intern Med. 2020;180(7):1–11. https://doi.org/10.1001/jamainternmed.2020.0994.
CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)-united states, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–6. https://doi.org/10.15585/mmwr.mm6912e2.
Wu YC, Chen CS, Chan YJ. The outbreak of COVID-19: an overview. J Chin Med Assoc. 2020;83(3):217–20. https://doi.org/10.1097/JCMA.0000000000000270.
Ahn DG, Shin HJ, Kim MH, Lee S, Kim HS, Myoung J, et al. Current status of epidemiology, diagnosis, therapeutics, and vaccinesfor novel coronavirus disease 2019 (COVID-19). J Microbiol Biotechnol. 2020;30(3):313–24. https://doi.org/10.4014/jmb.2003.03011.
Netea MG, van der Meer JW, van Crevel R. BCG vaccination in healthcare providers and the protection against COVID-19. J Clin Invest. 2020. https://doi.org/10.1172/JCI145545.
Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Netw Open. 2020;3(12):e2029058. https://doi.org/10.1001/jamanetworkopen.2020.29058.
Shamshirian A, Hessami A, Heydari K, Navaei RA, Ebrahimzadeh MA, Yip GW, et al. the role of hydroxychloroquine in COVID-19: a systematic review and meta-analysis. Ann Acad Med Singap. 2020;49(10):789–800.
Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, Tesoriero J, et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020;323(24):2493–502. https://doi.org/10.1001/jama.2020.8630.
Mitjà O, Corbacho-Monné M, Ubals M, et al. A cluster-randomized trial of hydroxychloroquine for prevention of Covid-1. N Engl J Med. 2020. https://doi.org/10.1056/NEJMoa2021801.10.1056/NEJMoa2021801.
Barnabas RV, Brown ER, Bershteyn A, Stankiewicz Karita HC, Johnston C, Thorpe LE, et al. Hydroxychloroquine as postexposure prophylaxis to prevent severe acute respiratory syndrome coronavirus 2 infection: a randomized trial. Ann Intern Med. 2020. https://doi.org/10.7326/M20-6519.10.7326/M20-6519.
Chen CP, Lin YC, Chen TC, Tseng TY, Wong HL, Kuo CY, et al. A multicenter, randomized, open-label, controlled trial to evaluate the efficacy and tolerability of hydroxychloroquine and a retrospective study in adult patients with mild to moderate coronavirus disease 2019 (COVID-19). PLoS ONE. 2020;15(12):e0242763. https://doi.org/10.1371/journal.pone.0242763 (eCollection 2020).
Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, et al. Repurposed antiviral drugs for covid-19: interim WHO solidarity trial results. N Engl J Med. 2020. https://doi.org/10.1056/NEJMoa2023184.
Cantini F, Goletti D, Petrone L, Najafi Fard S, Niccoli L, Foti R. Immune therapy, or antiviral therapy, or both for COVID-19: a systematic review. Drugs. 2020;80(18):1929–46. https://doi.org/10.1007/s40265-020-01421-w.
Bronte V, Ugel S, Tinazzi E, Vella A, De Sanctis F, Canè S, et al. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J Clin Invest. 2020;130(12):6409–16. https://doi.org/10.1172/JCI141772.
Rodriguez-Garcia JL, Sanchez-Nievas G, Arevalo-Serrano J, Garcia-Gomez C, Jimenez-Vizuete JM, Martinez-Alfaro E. Baricitinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: an observational cohort study. Rheumatol (Oxf). 2020. https://doi.org/10.1093/rheumatology/keaa587.
Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2020. https://doi.org/10.1056/NEJMoa2031994.10.1056/NEJMoa2031994.
Dai W, Wu J, Li T, Shen J, Pang R, Luo T, et al. Clinical outcomes for COVID-19 patients with diabetes mellitus treated with convalescent plasma transfusion in Wuhan. China J Med Virol. 2020. https://doi.org/10.1002/jmv.26712.10.1002/jmv.26712.
Alsharidah S, Ayed M, Ameen RM, Alhuraish F, Rouheldeen NA, Alshammari FR, et al. COVID-19 convalescent plasma treatment of moderate and severe cases of SARS-CoV-2 infection: a multicenter interventional study. Int J Infect Dis. 2020;S1201–9712(20):32513–23. https://doi.org/10.1016/j.ijid.2020.11.198.
Khalili M, Karamouzian M, Nasiri N, Javadi S, Mirzazadeh A, Sharifi H. Epidemiological characteristics of COVID-19: a systematic review and meta-analysis. Epidemiol Infect. 2020;148:e130. https://doi.org/10.1017/S0950268820001430.
McGonagle D, Sharif K, O’Regan A, Bridgewood C. Interleukin-6 use in COVID-19 pneumonia related macrophage activation syndrome. Autoimmun Rev. 2020. https://doi.org/10.1016/j.autrev.2020.102537.
Felsenstein S, Herbert JA, McNamara PS, Hedrich CM. COVID-19: immunology and treat- ment options. Clin Immunol. 2020;215:108448. https://doi.org/10.1016/j.clim.2020.108448.
Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, et al. Clinical features of 85 fatal cases of COVID-19 from wuhan: a retrospective observational study. Am J Respir Crit Care Med. 2020;201(11):1372–9. https://doi.org/10.1164/rccm.202003-0543OC.
Market M, Angka L, Martel AB, Bastin D, Olanubi O, Tennakoon G, et al. Flattening the COVID-19 curve with natural killer cell based immunotherapies. Front Immunol. 2020;11:1512. https://doi.org/10.3389/fimmu.2020.01512.
Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;26:842–4. https://doi.org/10.1038/s41591-020-0901-9.
Demaria O, Carvelli J, Batista L, Thibult ML, Morel A, André P, et al. Identification of druggable inhibitory immune checkpoints on natural killer cells in COVID-19. Cell Mol Immunol. 2020. https://doi.org/10.1038/s41423-020-0493-9.
Jiang M, Guo Y, Luo Q, Huang Z, Zhao R, Liu S, et al. T-Cell subset counts in peripheral blood can be used as discriminatory biomarkers for diagnosis and severity prediction of coronavirus disease 2019. J Infect Dis. 2020;222(2):198–202. https://doi.org/10.1093/infdis/jiaa252.
Sun DW, Zhang D, Tian RH, Li Y, Wang YS, Cao J, et al. The underlying changes and predicting role of peripheral blood inflammatory cells in severe COVID-19 patients: a sentinel? Clin Chim Acta. 2020;508:122–9. https://doi.org/10.1016/j.cca.2020.05.027.
Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17(5):533–5. https://doi.org/10.1038/s41423-020-0402-2.
Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol. 2015;33:643–75. https://doi.org/10.1146/annurev-immunol-032414-112220.
Park MD. Macrophages: a Trojan horse in COVID-19? Nat Rev Immunol. 2020;20(6):351. https://doi.org/10.1038/s41577-020-0317-2.
Bao L, Deng W, Huang B, Gao H, Liu J, Ren L, et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020. https://doi.org/10.1038/s41586-020-2312-y.10.1038/s41586-020-2312-y.
Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15(5):700–4. https://doi.org/10.1016/j.jtho.2020.02.010.
Wang C, Xie J, Zhao L, Fei X, Zhang H, Tan Y, et al. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients. EBioMedicine. 2020;57:102833. https://doi.org/10.1016/j.ebiom.2020.102833.
Chang YS, Ko BH, Ju JC, Chang HH, Huang SH, Lin CW. SARS unique domain (SUD) of Severe acute respiratory syndrome coronavirus induces NLRP3 Inflammasome-dependent CXCL10 -mediated pulmonary inflammation. Int J Mol Sci. 2020;21(9):3179. https://doi.org/10.3390/ijms21093179.
Pagliaro P. Is macrophages heterogeneity important in determining COVID-19 lethality? Med Hypotheses. 2020;143:110073. https://doi.org/10.1016/j.mehy.2020.110073.
Roney K. Bone marrow-derived dendritic cells. Methods Mol Biol. 2019;1960:57–62. https://doi.org/10.1007/978-1-4939-9167-9_4.
Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. https://doi.org/10.1007/3-540-32636-7_2.
Sanchez-Cerrillo I, Landete P, Aldave B, Sanchez-Alonso S, Sanchez-Azofra A, Marcos-Jimenez A, et al. Differential redistribution of activated monocyte and dendritic cell subsets to the lung associates with severity of COVID-19. Medrxiv. 2020. https://doi.org/10.1101/2020.05.13.20100925 (Preprint 2020;2020. 05.13. 20100925).
Yang D, Chu H, Hou Y, Chai Y, Shuai H, Lee AC, et al. Attenuated interferon and pro-inflammatory response in SARS-CoV-2-infected human dendritic cells is associated with viral antagonism of STAT1 phosphorylation. J Infect Dis. 2020. https://doi.org/10.1093/infdis/jiaa356.
Wei LL, Wang WJ, Chen DX, Xu B. Dysregulation of the immune response affects the outcome of critical COVID-19 patients. J Med Virol. 2020. https://doi.org/10.1002/jmv.26181.10.1002/jmv.26181.
Mitchell AJ, Roediger B, Weninger W. Monocyte homeostasis and the plasticity of inflammatory monocytes. Cell Immunol. 2014;291(1–2):22–31. https://doi.org/10.1016/j.cellimm.2014.05.010.
Peng J, Qi D, Yuan G, Deng X, Mei Y, Feng L, et al. Diagnostic value of peripheral hematologic markers for coronavirus disease 2019 (COVID-19): A multicenter, cross-sectional study. J Clin Lab Anal. 2019;2020:e23475. https://doi.org/10.1002/jcla.23475.
Gatti A, Radrizzani D, Viganò P, Mazzone A, Brando B. Decrease of non-classical and intermediate monocyte subsets in severe acute SARS-CoV-2 infection. Cytometry A. 2020. https://doi.org/10.1002/cyto.a.24188.10.1002/cyto.a.24188.
Ognibene A, Lorubbio M, Magliocca P, Tripodo E, Vaggelli G, Iannelli G, et al. Elevated monocyte distribution width in COVID-19 patients: the contribution of the novel sepsis indicator. Clin Chim Acta. 2020;509:22–4. https://doi.org/10.1016/j.cca.2020.06.002.
Zeng F, Li L, Zeng J, Deng Y, Huang H, Chen B, et al. Can we predict the severity of coronavirus disease 2019 with a routine blood test? Pol Arch Intern Med. 2020;130(5):400–6. https://doi.org/10.20452/pamw.15331.
Pence BD. Severe COVID-19 and aging: are monocytes the key? Geroscience. 2020. https://doi.org/10.1007/s11357-020-00213-0.
Xiong X, Chua GT, Chi S, Kwan MYW, Wong WHS, Zhou A, et al. Haematological and immunological data of Chinese children infected with coronavirus disease 2019. Data Brief. 2020;31:105953. https://doi.org/10.1016/j.dib.2020.105953.
Schönrich G, Raftery MJ. Neutrophil extracellular traps go viral. Front Immunol. 2016;7:366. https://doi.org/10.3389/fimmu.2016.00366.
Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18(2):134–47. https://doi.org/10.1038/nri.2017.105.
Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15(11):1318–21. https://doi.org/10.1038/nm.2053.
Fuchs TA, Brill A, Wagner DD. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2012;32(8):1777–83. https://doi.org/10.3389/fimmu.2018.00288.
Zhou Y, Guo S, He Y, Zuo Q, Liu D, Xiao M, et al. COVID-19 is distinct from SARS-CoV-2-negative community-acquired pneumonia. Front Cell Infect Microbiol. 2020;10:322. https://doi.org/10.3389/fcimb.2020.00322.
Xia XY, Wu J, Liu HL, Xia H, Jia B, Huang WX. Epidemiological and initial clinical characteristics of patients with family aggregation of COVID-19. J Clin Virol. 2020;127:104360. https://doi.org/10.1016/j.jcv.2020.104360.
Wu H, Zhu H, Yuan C, Yao C, Luo W, Shen X, et al. Clinical and immune features of hospitalized pediatric patients with coronavirus disease 2019 (COVID-19) in Wuhan, China. JAMA Netw Open. 2020;3(6):e2010895. https://doi.org/10.1001/jamanetworkopen.2020.10895.
Liu J, Liu Y, Xiang P, Pu L, Xiong H, Li C, et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med. 2020;18(1):206. https://doi.org/10.1186/s12967-020-02374-0.
Xia X, Wen M, Zhan S, He J, Chen W. An increased neutrophil/lymphocyte ratio is an early warning signal of severe COVID-19. Nan Fang Yi Ke Da Xue Xue Bao. 2020;40(3):333–6. https://doi.org/10.12122/j.issn.1673-4254.2020.03.06.
Wang H, Zhang Y, Mo P, Liu J, Wang H, Wang F, et al. Neutrophil to CD4+ lymphocyte ratio as a potential biomarker in predicting virus negative conversion time in COVID-19. Int Immunopharmacol. 2020;85:106683. https://doi.org/10.1016/j.intimp.2020.106683.
Didangelos A. COVID-19 Hyperinflammation: What about Neutrophils? mSphere. 2020;5(3):e00367-e420. https://doi.org/10.1128/mSphere.00367-20.
Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5(11):e138999. https://doi.org/10.1172/jci.insight.138999.
Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med. 2020;217(6):e20200652. https://doi.org/10.1084/jem.20200652.
Marshall JS, Portales-Cervantes L, Leong E. Mast cell responses to viruses and pathogen products. Int J Mol Sci. 2019;20(17):4241. https://doi.org/10.3390/ijms20174241 (Published 2019 Aug 30).
Afrin LB, Weinstock LB, Molderings GJ. Covid-19 hyperinflammation and post-Covid-19 illness may be rooted in mast cell activation syndrome. Int J Infect Dis. 2020;100:327–32. https://doi.org/10.1016/j.ijid.2020.09.016.
Conti P, Caraffa A, Tetè G, Gallenga CE, Ross R, Kritas SK, et al. Mast cells activated by SARS-CoV-2 release histamine which increases IL-1 levels causing cytokine storm and inflammatory reaction in COVID-19. J Biol Regul Homeost Agents. 2020;34(5):1629–32. https://doi.org/10.23812/20-2EDIT.
Theoharides TC. Potential association of mast cells with COVID-19. Ann Allergy Asthma Immunol. 2020;S1081–1206(20):31165. https://doi.org/10.1016/j.anai.2020.11.003.
Chen X, Liu S, Goraya MU, Maarouf M, Huang S, Chen JL. Host immune response to influenza a virus infection. Front Immunol. 2018;9:320. https://doi.org/10.3389/fimmu.2018.00320.
Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–9. https://doi.org/10.1172/JCI137244.
Liu J, Li S, Liu J, Liang B, Wang X, Wang H, Li W, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. https://doi.org/10.1016/j.ebiom.2020.102763.
Vardhana SA, Wolchok JD. The many faces of the anti-COVID immune response. J Exp Med. 2020;217(6):e20200678. https://doi.org/10.1084/jem.20200678.
Liu B, Han JY, Cheng XH, Yu L, Zhang L, Wang W, et al. Persistent SARS-CoV-2 presence is companied with defects in adaptive immune system in non-severe COVID-19 patients. medRxiv. 2020. https://doi.org/10.1101/2020.03.26.20044768 (Preprint).
Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–2. https://doi.org/10.1016/S2213-2600(20)30076-X.
Le Bert N, Tan AT, Kunasegaran K, Tham CYL, Hafezi M, Chia A, et al. SARS-CoV-2 -specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020. https://doi.org/10.1038/s41586-020-2550-z.10.1038/s41586-020-2550-z.
Ni L, Ye F, Cheng ML, Feng Y, Deng YQ, Zhao H, et al. Detection of SARS-CoV-2-Specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020;52(6):971-977.e3. https://doi.org/10.1016/j.immuni.2020.04.023.
He R, Lu Z, Zhang L, Fan T, Xiong R, Shen X, et al. The clinical course and its correlated immune status in COVID-19 pneumonia. J Clin Virol. 2020;127:104361. https://doi.org/10.1016/j.jcv.2020.104361.
Xu B, Fan CY, Wang AL, Zou YL, Yu YH, He C, et al. Suppressed T cell-mediated immunity in patients with COVID-19: a clinical retrospective study in Wuhan. China J Infect. 2020;81(1):e51–60. https://doi.org/10.1016/j.jinf.2020.04.012.
Schultheiß C, Paschold L, Simnica D, Mohme M, Willscher E, von Wenserski L, et al. Next-generation sequencing of T and B Cell receptor repertoires from COVID-19 patients showed signatures associated with severity of disease. Immunity. 2020. https://doi.org/10.1016/j.immuni.2020.06.024.
Ju B, Zhang Q, Ge J, Wang R, Sun J, Ge X, et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020. https://doi.org/10.1038/s41586-020-2380-z.10.1038/s41586-020-2380-z.
Amanat F, Stadlbauer D, Strohmeier S, Nguyen THO, Chromikova V, McMahon M, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26(7):1033–6. https://doi.org/10.1038/s41591-020-0913-5.
Seow J, Graham C, Merrick B, Acors S, Steel K, Hemmings O, et al. Longitudinal evaluationand declineof antibody responses in SARS-CoV-2 infection. Preprint medRxiv. 2020. https://doi.org/10.1101/2020.07.09.20148429.
Juno JA, Tan HX, Lee WS, Reynaldi A, Kelly HG, Wragg K, et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat Med. 2020. https://doi.org/10.1038/s41591-020-0995-0.
Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthriti. Lancet. 2016;388(10055):1984. https://doi.org/10.1016/S0140-6736(16)30173-8.
Schett G, Manger B, Simon D, Caporali R. COVID-19 revisiting inflammatory pathways of arthritis. Nat Rev Rheumatol. 2020;16(8):465–70. https://doi.org/10.1038/s41584-020-0451-z.
Ji W, Huh K, Kang M, Hong J, Bae GH, Lee R, et al. Effect of underlying comorbidities on the infection and severity of COVID-19 in Korea: a nationwide case-control study. J Korean Med Sci. 2020;35(25):e237. https://doi.org/10.3346/jkms.2020.35.e237.
Taylor PC. Adopting PROs in virtual and outpatient management of RA. Nat Rev Rheumatol. 2020;16(9):477–8. https://doi.org/10.1038/s41584-020-0449-6.
Mistry J, Sharif M, Prideaux A, Smith C, Sumbwanyambe M, Sibley M, et al. Use of rheumatoid arthritis impact of disease (RAID) in routine care; identification of DAS28 remission and unmet patient reported outcomes. Rheumatol Adv Pract. 2020;4(2):013. https://doi.org/10.1093/rap/rkaa013.
Ferreira RJO, Carvalho PD, Ndosi M, Duarte C, Chopra A, Murphy E, et al. Impact of patient’s global assessment on achieving remission in patients with rheumatoid arthritis: a multinational study using the Meteor database. Arthritis Care Res (Hoboken). 2019;71(10):1317–25. https://doi.org/10.1002/acr.23866.
Bugatti S, De Stefano L, Favalli EG, Caporali R. Increasing the threshold for patient global assessment in defining remission may have a different impact in patients with early and established rheumatoid arthritis. Ann Rheum Dis. 2020. https://doi.org/10.1136/annrheumdis-2020-217488 (annrheumdis-2020-217488).
Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet. 2014;384(1878–8):8. https://doi.org/10.1016/S0140-6736(14)60128-8.
Holubar J, Le Quintrec M, Letaief H, Faillie JL, Pers YM, Jorgensen C. Monitoring of patients with systemic lupus erythematosus during the COVID-19 outbreak. Ann Rheum Dis. 2020. https://doi.org/10.1136/annrheumdis-2020-217919 (annrheumdis-2020-217919).
Favalli EG, Gerosa M, Murgo A, Caporali R. Are patients with systemic lupus erythematosus at increased risk for COVID-19? Ann Rheum Dis. 2020. https://doi.org/10.1136/annrheumdis-2020-217787 (annrheumdis-2020-217787).
Pablos JL, Abasolo L, Alvaro-Gracia JM, Blanco FJ, Blanco R, Castrejón I, et al. Prevalence of hospital PCR-confirmed COVID-19 cases in patients with chronic inflammatory and autoimmune rheumatic diseases. Ann Rheum Dis. 2020. https://doi.org/10.1136/annrheumdis-2020-217763 (annrheumdis-2020-217763).
Fernandez-Ruiz R, Masson M, Kim MY, Myers B, Haberman RH, Castillo R, et al. Leveraging the United States epicenter to provide insights on COVID-19 in patients with systemic lupus erythematosus. Arthritis Rheumatol. 2020. https://doi.org/10.1002/art.41450.10.1002/art.41450.
Gendebien Z, von Frenckell C, Ribbens C, André B, Thys M, Gangolf M, et al. Systematic analysis of COVID-19 infection and symptoms in a systemic lupus erythematosus population: correlation with disease characteristics, hydroxychloroquine use and immunosuppressive treatments. Ann Rheum Dis. 2020. https://doi.org/10.1136/annrheumdis-2020-218244 (annrheumdis-2020-218244).
Wallace B, Washer L, Marder W, Kahlenberg JM. Patients with lupus with COVID-19: university of Michigan experience. Ann Rheum Dis. 2020. https://doi.org/10.1136/annrheumdis-2020-217794 (annrheumdis-2020-217794).
Sawalha AH, Zhao M, Coit P, Lu Q. Epigenetic dysregulation of ACE2 and interferon -regulated genes might suggest increased COVID-19 susceptibility and severity in lupus patients. Clin Immunol. 2020;215:108410. https://doi.org/10.1016/j.clim.2020.108410.
Konig MF, Kim AH, Scheetz MH, Graef ER, Liew JW, Simard J, et al. COVID-19 global rheumatology alliance baseline. use of hydroxychloroquine in systemic lupus erythematosus does not preclude SARS-CoV-2 infection and severe COVID-19. Ann Rheum Dis. 2020. https://doi.org/10.1136/annrheumdis-2020-217690 (annrheumdis-2020-217690).
Mathian A, Mahevas M, Rohmer J, Roumier M, Cohen-Aubart F, Amador-Borrero B, et al. Clinical course of coronavirus disease 2019 (COVID-19) in a series of 17 patients with systemic lupus erythematosus under long-term treatment with hydroxychloroquine. Ann Rheum Dis. 2020;79(6):837–9. https://doi.org/10.1136/annrheumdis-2020-217566.
Horisberger A, Moi L, Ribi C, Comte D. Impact of COVID-19 pandemic on SLE: beyond the risk of infection. Lupus Sci Med. 2020;7(1):e000408. https://doi.org/10.1136/lupus-2020-000408.
Ramagopalan SV, Dobson R, Meier UC, Giovannoni G. Multiple sclerosis: risk factors, prodromes, and potential causal pathways. Lancet Neurol. 2010;9(7):727–39. https://doi.org/10.1016/S1474-4422(10)70094-6.
Castillo Álvarez F, López Pérez MÁ, Marzo Sola ME. Risk of SARS-CoV-2 infection and clinical outcomes in multiple sclerosis patients in La Rioja (Spain). Med Clin (Barc). 2020;S0025–7753(20):30444–9. https://doi.org/10.1016/j.medcli.2020.06.021.
Louapre C, Collongues N, Stankoff B, Giannesini C, Papeix C, Bensa C, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77(9):1079–88. https://doi.org/10.1001/jamaneurol.2020.2581.
Crescenzo F, Marastoni D, Bovo C, Calabrese M. Frequency and severity of COVID-19 in multiple sclerosis: a short single-site report from northern Italy. Mult Scler Relat Disord. 2020;44:102372. https://doi.org/10.1016/j.msard.2020.102372.
Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature. 2020. https://doi.org/10.1038/s41586-020-2521-4.
Cristiano E, Patrucco L, Rojas JI, Nuñez S. Estimating the risk of COVID-19 in multiple sclerosis patients in Buenos Aires. Argentina Mult Scler Relat Disord. 2020;44:102307. https://doi.org/10.1016/j.msard.2020.102307.
Fan M, Qiu W, Bu B, Xu Y, Yang H, Huang D, et al. Risk of COVID-19 infection in MS and neuromyelitis optica spectrum disorders. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e787. https://doi.org/10.1212/NXI.0000000000000787.
Aguirre C, Meca-Lallana V, Barrios-Blandino A, Del Río B, Vivancos J. Covid-19 in a patient with multiple sclerosis treated with natalizumab: may the blockade of integrins have a protective role? Mult Scler Relat Disord. 2020;44:102250. https://doi.org/10.1016/j.msard.2020.102250.
Guevara C, Villa E, Rosas CS, Diaz V, Naves R. Treating patients with multiple sclerosis during the COVID-19 pandemic: assessing the expert recommendations. Mult Scler Relat Disord. 2020;43:102224. https://doi.org/10.1016/j.msard.2020.102224.
Safavi F, Nourbakhsh B, Azimi AR. B-cell depleting therapies may affect susceptibility to acute respiratory illness among patients with multiple sclerosis during the early COVID-19 epidemic in Iran. Mult Scler Relat Disord. 2020;43:102195. https://doi.org/10.1016/j.msard.2020.102195.
Ghajarzadeh M, Mirmosayyeb O, Barzegar M, Nehzat N, Vaheb S, Shaygannejad V, et al. Favorable outcome after COVID-19 infection in a multiple sclerosis patient initiated on ocrelizumab during the pandemic. Mult Scler Relat Disord. 2020;43:102222. https://doi.org/10.1016/j.msard.2020.102222.
Montero-Escribano P, Matías-Guiu J, Gómez-Iglesias P, Porta-Etessam J, Pytel V, Matias-Guiu JA. Anti-CD20 and Covid-19 in multiple sclerosis and related disorders: a case series of 60 patients from madrid. Spain Mult Scler Relat Disord. 2020;42:102185. https://doi.org/10.1016/j.msard.2020.102185.
Hodson R. Inflammatory bowel disease. Nature. 2016;540(7634):S97. https://doi.org/10.1038/540S9a.
Aziz M, Fatima R, Haghbin H, Lee-Smith W, Nawras A. The incidence and outcomes of COVID -19 in IBD patients: a rapid review and meta-analysis. Inflamm Bowel Dis. 2020. https://doi.org/10.1093/ibd/izaa170.
Grunert PC, Reuken PA, Stallhofer J, Teich N, Stallmach A. Inflammatory bowel disease in the COVID-19 pandemic-the patients’ perspective. J Crohns Colitis. 2020. https://doi.org/10.1093/ecco-jcc/jjaa126.
Zingone F, Savarino EV. Viral screening before initiation of biologics in patients with inflammatory bowel disease during the COVID-19 outbreak. Lancet Gastroenterol Hepatol. 2020;5(6):525. https://doi.org/10.1016/S2468-1253(20)30085-6.
Allocca M, Fiorino G, Zallot C, Furfaro F, Gilardi D, Radice S, et al. Incidence and patterns of COVID-19 among inflammatory bowel disease patients from the nancy and milan cohorts. Clin Gastroenterol Hepatol. 2020;18(9):2134–5. https://doi.org/10.1016/j.cgh.2020.04.071.
Gubatan J, Levitte S, Balabanis T, Patel A, Sharma A, Habtezion A. SARS-CoV-2 testing, prevalence, and predictors of COVID-19 in patients with inflammatory bowel disease in northern california. Gastroenterology. 2020;S0016–5085(20):30601–6. https://doi.org/10.1053/j.gastro.2020.05.009.
Singh S, Khan A, Chowdhry M, Bilal M, Kochhar GS, Clarke K. Risk of severe COVID-19 in patients with inflammatory bowel disease in united states. Multicent Res Netw Study Gastroenterol. 2020;S0016–5085(20):34755–7. https://doi.org/10.1053/j.gastro.2020.06.003.
Axelrad JE, Malter L, Hong S, Chang S, Bosworth B, Hudesman D. From the American epicenter: coronavirus disease 2019 in patients with inflammatory bowel disease in the New York city metropolitan area. Inflamm Bowel Dis. 2020. https://doi.org/10.1093/ibd/izaa162.
Lukin DJ, Kumar A, Hajifathalian K, Sharaiha RZ, Scherl EJ, Longman RS, et al. Baseline disease activity and steroid therapy stratify risk of COVID-19 in patients with inflammatory bowel disease. Gastroenterology. 2020;S0016–5085(20):34738–47. https://doi.org/10.1053/j.gastro.2020.05.066.
Krzysztof NJ, Christoffer LJ, Rahul K, Ricanek P, Jonas H, Jack S. Age, inflammation and disease location are critical determinants of intestinal expression of SARS-CoV-2 receptor ACE2 and TMPRSS2 in inflammatory bowel disease. Gastroenterology. 2020;S0016–5085(20):30653–63. https://doi.org/10.1053/j.gastro.2020.05.030.
Kennedy NA, Jones GR, Lamb CA, Appleby R, Arnott I, Beattie RM, et al. British society of gastroenterology guidance for management of inflammatory bowel disease during the COVID-19 pandemic. Gut. 2020;69(6):984–90. https://doi.org/10.1136/gutjnl-2020-321244.
Rubin DT, Feuerstein JD, Wang AY, Cohen RD. AGA clinical practice update on management of inflammatory bowel disease during the COVID-19 pandemic: expert commentary. Gastroenterology. 2020;159(1):350–7. https://doi.org/10.1053/j.gastro.2020.04.012.
Hanzel J, Ma C, Marshall JK, Feagan BG, Jairath V. Managing inflammatory bowel disease during COVID-19: summary of recommendations from gastrointestinal societies. Clin Gastroenterol Hepatol. 2020;18(9):2143–6. https://doi.org/10.1016/j.cgh.2020.04.033.
Allocca M, Fiorino G, Furfaro F, Gilardi D, Radice S, D’Amico F, et al. Maintaining the quality standards of care for inflammatory bowel disease patients during the COVID-19 pandemic. Clin Gastroenterol Hepatol. 2020;18(8):1882–3. https://doi.org/10.1016/j.cgh.2020.04.028.
Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464(7293):1293–300. https://doi.org/10.1038/nature08933.
Vangoitsenhoven R, Martens PJ, van Nes F, Moyson C, Nobels F, Van Crombrugge P, et al. No evidence of increased hospitalization rate for COVID-19 in community-dwelling patients with type 1 diabetes. Diabetes Care. 2020. https://doi.org/10.2337/dc20-1246.
Capaldo B, Annuzzi G, Creanza A, Giglio C, De Angelis R, Lupoli R, et al. Blood glucose control during lockdown for COVID-19: CGM metrics in italian adults with type 1 diabetes. Diabetes Care. 2020;43(8):e88–9. https://doi.org/10.2337/dc20-1127.
Tornese G, Ceconi V, Monasta L, Carletti C, Faleschini E, Barbi E. Glycemic control in type 1 diabetes mellitus during COVID-19 quarantine and the role of in-home physical activity. Diabetes Technol Ther. 2020;22(6):462–7. https://doi.org/10.1089/dia.2020.0169.
Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43(8):e83–5. https://doi.org/10.2337/dc20-1088.
Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390(10103):1685–99. https://doi.org/10.1016/S0140-6736(17)30933-9.
Matucci-Cerinic M, Bruni C, Allanore Y, Clementi M, Dagna L, Damjanov NS, et al. Systemic sclerosis and the COVID-19 pandemic: world scleroderma foundation preliminary advice for patient management. Ann Rheum Dis. 2020;79(6):724–6. https://doi.org/10.1136/annrheumdis-2020-217407.
Minniti A, Maglione W, Pignataro F, Cappadona C, Caporali R, Del Papa N. Taking care of systemic sclerosis patients during COVID-19 pandemic: rethink the clinical activity. Clin Rheumatol. 2020;39(7):2063–5. https://doi.org/10.1007/s10067-020-05191-4.
Avouac J, Airó P, Carlier N, Matucci-Cerinic M, Allanore Y. Severe COVID-19-associated pneumonia in 3 patients with systemic sclerosis treated with rituximab. Ann Rheum Dis. 2020. https://doi.org/10.1136/annrheumdis-2020-217864.
Smith JA. Update on ankylosing spondylitis: current concepts in pathogenesis. Curr Allergy Asthma Rep. 2015;15(1):489. https://doi.org/10.1007/s11882-014-0489-6.
Coskun Benlidayi I, Kurtaran B, Tirasci E, Guzel R. Coronavirus disease 2019 (COVID-19) in a patient with ankylosing spondylitis treated with secukinumab: a case-based review. Rheumatol Int. 2019;2020:1–10. https://doi.org/10.1007/s00296-020-04635-z.
Lee JM, Lee SJ. Olfactory and gustatory dysfunction in a COVID-19 patient with ankylosing spondylitis treated with etanercept: case report. J Korean Med Sci. 2020;35(21):e201. https://doi.org/10.3346/jkms.2020.35.e201.
Rendon A, Schäkel K. Psoriasis pathogenesis and treatment. Int J Mol Sci. 2019;20(6):1475. https://doi.org/10.1016/S0140-6736(14)61909-7.
Akiyama S, Hamdeh S, Micic D, Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis. 2020. https://doi.org/10.1136/annrheumdis-2020-218946.
Torres T, Puig L. Managing cutaneous immune-mediated diseases during the COVID-19 pandemic. Am J Clin Dermatol. 2020;21(3):307–11. https://doi.org/10.1007/s40257-020-00514-2.
Lebwohl M, Rivera-Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID-19? J Am Acad Dermatol. 2020;82(5):1217–8. https://doi.org/10.1016/j.jaad.2020.03.031.
Gisondi P, Bellinato F, Chiricozzi A, Girolomoni G. The risk of COVID-19 pandemic in patients with moderate to severe plaque psoriasis receiving systemic treatments. Vaccines (Basel). 2020;8(4):E728. https://doi.org/10.3390/vaccines8040728.
Annapureddy N, Nalleballe K, Onteddu SR, Sharma R, Sheng S, Kovvuru S, et al. Biologics in systemic autoimmune diseases during COVID-19 pandemic. Clin Rheumatol. 2020;39(12):3529–31. https://doi.org/10.1007/s10067-020-05439-z.
Ferri C, Giuggioli D, Raimondo V, L’Andolina M, Tavoni A, Cecchetti R, et al. COVID-19 and rheumatic autoimmune systemic diseases: report of a large Italian patients series. Clin Rheumatol. 2020;39(11):3195–204. https://doi.org/10.1007/s10067-020-05334-7.
Gianfrancesco M, Hyrich KL, Al-Adely S, Carmona L, Danila MI, Gossec L, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis. 2020;79(7):859–66. https://doi.org/10.1136/annrheumdis-2020-217871.
Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. https://doi.org/10.1371/journal.pmed.0030343.
Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA, et al. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757–67. https://doi.org/10.1164/rccm.201706-1172OC.
WHO. COVID-19 Situation Report (2020). Weekly Operational Update on COVID-19. 4 Spetember 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/wou-4-september-2020 -approved. pdf?sfvrsn=91215c78_2 (assessed 7 September 2020).
Dubbioso R, Nobile-Orazio E, Manganelli F, Santoro L, Briani C, Cocito D, et al. Dealing with immune-mediated neuropathies during COVID-19 outbreak: practical recommendations from the task force of the Italian society of neurology (SIN), the Italian society of clinical neurophysiology (SINC) and the Italian peripheral nervous system association (ASNP). Neurol Sci. 2020;41(6):1345–8. https://doi.org/10.1007/s10072-020-04448-9.
Ceribelli A, Motta F, De Santis M, et al. Recommendations for coronavirus infection in rheumatic diseases treated with biologic therapy. J Autoimmun. 2020;109:102442. https://doi.org/10.1016/j.jaut.2020.102442.
Au K, Reed G, Curtis JR, Kremer JM, Greenberg JD, Strand V, et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(5):785–91. https://doi.org/10.1136/ard.2010.128637.
Ciotti JR, Valtcheva MV, Cross AH. Effects of MS disease-modifying therapies on responses to vaccinations: a review. Mult Scler Relat Disord. 2020;45:102439. https://doi.org/10.1016/j.msard.2020.102439.
Houot R, Levy R, Cartron G, Armand P. Could anti-CD20 therapy jeopardise the efficacy of a SARS-CoV-2 vaccine? Eur J Cancer. 2020;136:4–6. https://doi.org/10.1016/j.ejca.2020.06.017.
Baker D, Roberts CAK, Pryce G, Kang AS, Marta M, Reyes S, et al. COVID-19 vaccine- readiness for anti-CD20-depleting therapy in autoimmune diseases. Clin Exp Immunol. 2020;202(2):149–61. https://doi.org/10.1111/cei.13495.
Acknowledgements
This work were partly supported by grants from National Medical Professional Degree Graduate Education Steering Committee (code: B2-YX20190203-01), Excellent Open Courses for Graduate Students of Anhui Medical University (code: yjsjpkc201901), and Key Project of the Education Department of Anhui Province Natural Science Research (code: KJ2017A164), Anhui provincial laboratory of population health and major disease screening and diagnosis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, J., Liu, HH., Yin, XD. et al. COVID-19 illness and autoimmune diseases: recent insights. Inflamm. Res. 70, 407–428 (2021). https://doi.org/10.1007/s00011-021-01446-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-021-01446-1