Abstract
Purpose
Triple-negative breast cancers (TNBC) are aggressive tumours that exhibit abundant lymphoid infiltrates which modulate tumour behaviour. Recent findings suggest that TNBC with higher densities of plasma cells are associated with a favourable prognosis, and tertiary lymphoid structures (TLS) have prognostic significance. Here, we studied the phenotype and function of plasma cells in TNBCs by assessing their association with IgG Kappa light chain expression, B cells, and TLS.
Methods
A retrospective analysis of 269 TNBC cases was performed. Tumour-infiltrating CD38+ plasma cells, CD20+ B cells, and TLS were evaluated on conventional haematoxylin–eosin-stained and immunohistochemical-stained sections of TNBC. We then selected TNBC cases demonstrating the highest and lowest densities of plasma cells, and examined their association with TLS, B cells, as well as immunoglobulin expression using Opal-Vectra multiplex immunofluorescence (IF).
Results
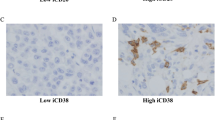
TNBC with high density of plasma cells showed significantly higher numbers of IgG Kappa+ CD38+ cells (p = 0.0089, p < 0.0001), and higher numbers of TLS (p < 0.0001), compared to TNBC with low density of plasma cells. TNBC with high density of plasma cells also showed higher numbers of CD20+ B cells in the tumour core (p < 0.0001), invasive margin (p < 0.0001), as well as stromal (p = 0.015) compartments.
Conclusion
TNBC with high density of plasma cells are associated with higher numbers of IgG Kappa+ CD38+ cells, CD20+ B cells, and TLS. Further studies to characterize the function of plasma cell infiltrates and how they may interact with other tumour-infiltrating lymphocytes and TLS in TNBC may help improve existing immunotherapy strategies.




Similar content being viewed by others
References
Foulkes WD, Smith IE, Reis-Filho JS (2010) Triple-negative breast cancer. N Engl J Med 363(20):1938–1948. https://doi.org/10.1056/NEJMra1001389
Thike AA, Yian Cheok P, Richelia Jara-Lazaro A, Tan B, Tan P, Hoon TP (2009) Triple-negative breast cancer: clinicopathological characteristics and relationship with basal-like breast cancer. Mod Pathol 23(1):123–133. https://doi.org/10.1038/modpathol.2009.145
Rakha EA, Reis-Filho JS, Ellis IO (2008) Basal-like breast cancer: a critical review. J Clin Oncol. https://doi.org/10.1200/JCO.2007.13.1748
Dent R, Trudeau M, Pritchard KI et al (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13(15):4429–4434. https://doi.org/10.1158/1078-0432.CCR-06-3045
Cleator S, Heller W, Coombes RC (2007) Triple-negative breast cancer: therapeutic options. Lancet Oncol. https://doi.org/10.1016/S1470-2045(07)70074-8
Hudis CA, Gianni L (2011) Triple-negative breast cancer: an unmet medical need. Oncologist 16(Supplement 1):1–11. https://doi.org/10.1634/theoncologist.2011-S1-01
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell. https://doi.org/10.1016/j.cell.2011.02.013
Yeong J, Thike AA, Lim JCT et al (2017) Higher densities of Foxp3+ regulatory T cells are associated with better prognosis in triple-negative breast cancer. Breast Cancer Res Treat 163(1):21–35. https://doi.org/10.1007/s10549-017-4161-4
Bottai G, Raschioni C, Losurdo A et al (2016) An immune stratification reveals a subset of PD-1/LAG-3 double-positive triple-negative breast cancers. Breast Cancer Res 18:121. https://doi.org/10.1186/s13058-016-0783-4
Denkert C, von Minckwitz G, Brase JC et al (2015) Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. https://doi.org/10.1200/JCO.2014.58.1967
Ono M, Tsuda H, Shimizu C et al (2012) Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat. https://doi.org/10.1007/s10549-011-1554-7
Miyashita M, Sasano H, Tamaki K et al (2015) Prognostic significance of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple-negative breast cancer: a retrospective multicenter study. Breast Cancer Res 17(1):124. https://doi.org/10.1186/s13058-015-0632-x
Loi S, Michiels S, Salgado R et al (2014) Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. https://doi.org/10.1093/annonc/mdu112
DeNardo DG, Brennan DJ, Rexhepaj E et al (2011) Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov 1(1):54–67. https://doi.org/10.1158/2159-8274.CD-10-0028
Schwartz M, Zhang Y, Rosenblatt JD (2016) B cell regulation of the anti-tumor response and role in carcinogenesis. J Immunother Cancer. https://doi.org/10.1186/s40425-016-0145-x
Zhang Y, Gallastegui N, Rosenblatt JD (2015) Regulatory B cells in anti-tumor immunity. Int Immunol. https://doi.org/10.1093/intimm/dxv034
Weiner LM, Dhodapkar MV, Ferrone S (2009) Monoclonal antibodies for cancer immunotherapy. Lancet. https://doi.org/10.1016/S0140-6736(09)60251-8.Monoclonal
Topalian SL, Weiner GJ, Pardoll DM (2011) Cancer immunotherapy comes of age. J Clin Oncol. https://doi.org/10.1200/JCO.2011.38.0899
Weiner LM, Murray JC, Shuptrine CW (2012) Antibody-based immunotherapy of cancer. Cell. https://doi.org/10.1016/j.cell.2012.02.034
Marsigliante S, Biscozzo L, Marra A et al (1999) Computerised counting of tumour infiltrating lymphocytes in 90 breast cancer specimens. Cancer Lett. https://doi.org/10.1016/S0304-3835(98)00379-6
Nelson BH (2010) CD20+ B Cells: the other tumor-infiltrating lymphocytes. J Immunol. https://doi.org/10.4049/jimmunol.1001323
Coronella-Wood JA, Hersh EM (2003) Naturally occurring B-cell responses to breast cancer. Cancer Immunol Immunother. https://doi.org/10.1007/s00262-003-0409-4
Brown JR, Wimberly H, Lannin DR, Nixon C, Rimm DL, Bossuyt V (2014) Multiplexed quantitative analysis of CD3, CD8, and CD20 predicts response to neoadjuvant chemotherapy in breast cancer. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-14-1622
Kroeger DR, Milne K, Nelson BH (2016) Tumor-infiltrating plasma cells are associated with tertiary lymphoid structures, cytolytic T-cell responses, and superior prognosis in ovarian cancer. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-15-2762
Berntsson J, Nodin B, Eberhard J, Micke P, Jirström K (2016) Prognostic impact of tumour-infiltrating B cells and plasma cells in colorectal cancer. Int J Cancer 139(5):1129–1139. https://doi.org/10.1002/ijc.30138
Ito T, Saga S, Nagayoshi S et al (1986) Class distribution of immunoglobulin-containing plasma cells in the stroma of medullary carcinoma of breast. Breast Cancer Res Treat 7(2):97–103
Mohammed ZMA, Going JJ, Edwards J, Elsberger B, Mcmillan DC (2013) The relationship between lymphocyte subsets and clinico-pathological determinants of survival in patients with primary operable invasive ductal breast cancer. Br J Cancer. https://doi.org/10.1038/bjc.2013.493
Wei H, Fu P, Yao M, Chen Y, Du L (2016) Breast cancer stem cells phenotype and plasma cell-predominant breast cancer independently indicate poor survival. Pathol Res Pract. https://doi.org/10.1016/j.prp.2016.01.008
Yeong J, Lim JCT, Lee B et al (2018) High densities of tumor-associated plasma cells predict improved prognosis in triple negative breast cancer. Front Immunol 9:1209
Alistar A, Chou JW, Nagalla S, Black MA, D’Agostino R, Miller LD (2014) Dual roles for immune metagenes in breast cancer prognosis and therapy prediction. Genome Med. https://doi.org/10.1186/s13073-014-0080-8
Hanker LC, Rody A, Holtrich U et al (2013) Prognostic evaluation of the B cell/IL-8 metagene in different intrinsic breast cancer subtypes. Breast Cancer Res Treat 137(2):407–416. https://doi.org/10.1007/s10549-012-2356-2
Dieu-Nosjean MC, Goc J, Giraldo NA, Sautès-Fridman C, Fridman WH (2014) Tertiary lymphoid structures in cancer and beyond. Trends Immunol. https://doi.org/10.1016/j.it.2014.09.006
Lee M, Heo S-H, Song IH et al (2019) Presence of tertiary lymphoid structures determines the level of tumor-infiltrating lymphocytes in primary breast cancer and metastasis. Mod Pathol 32(1):70–80. https://doi.org/10.1038/s41379-018-0113-8
Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ (2012) WHO classification of tumours of the breast, 4th edn. IARC, France
Thike AA, Chong LYZ, Cheok PY et al (2014) Loss of androgen receptor expression predicts early recurrence in triple-negative and basal-like breast cancer. Mod Pathol. https://doi.org/10.1038/modpathol.2013.145
Thike AA, Iqbal J, Cheok PY, Tse GM-K, Tan PH (2013) Ductal carcinoma in situ associated with triple negative invasive breast cancer: evidence for a precursor–product relationship. J Clin Pathol 66(8):665–670. https://doi.org/10.1136/jclinpath-2012-201428
Salgado R, Denkert C, Demaria S et al (2015) The evaluation of tumor-infiltrating lymphocytes (TILS) in breast cancer: recommendations by an International TILS Working Group 2014. Ann Oncol. https://doi.org/10.1093/annonc/mdu450
Herbst RS, Soria JC, Kowanetz M et al (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. https://doi.org/10.1038/nature14011
Sun X, Zhang T, Li M, Yin L, Xue J (2019) Immunosuppressive B cells expressing PD-1/PD-L1 in solid tumors: a mini review. QJM An Int J Med. https://doi.org/10.1093/qjmed/hcz162
Garnelo M, Tan A, Her Z et al (2017) Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut. https://doi.org/10.1136/gutjnl-2015-310814
Lim JCT, Yeong JPS, Lim CJ et al (2018) An automated staining protocol for seven-colour immunofluorescence of human tissue sections for diagnostic and prognostic use. Pathology. https://doi.org/10.1016/j.pathol.2017.11.087
Fiore C, Bailey D, Conlon N et al (2012) Utility of multispectral imaging in automated quantitative scoring of immunohistochemistry. J Clin Pathol. https://doi.org/10.1136/jclinpath-2012-200734
Buisseret L, Desmedt C, Garaud S et al (2017) Reliability of tumor-infiltrating lymphocyte and tertiary lymphoid structure assessment in human breast cancer. Mod Pathol 30(9):1204–1212. https://doi.org/10.1038/modpathol.2017.43
Lee HJ, Park IA, Song IH et al (2016) Tertiary lymphoid structures: prognostic significance and relationship with tumour-infiltrating lymphocytes in triple-negative breast cancer. J Clin Pathol 69(5):422–430
Wee YTF, Alkaff SMF, Lim JCT et al (2018) An integrated automated multispectral imaging technique that simultaneously detects and quantitates viral RNA and immune cell protein markers in fixed sections from Epstein-Barr virus-related tumours. Ann Diagn Pathol 37:12–19. https://doi.org/10.1016/j.anndiagpath.2018.09.002
Schmidt M, Micke P, Gehrmann M, Hengstler JG (2012) Immunoglobulin kappa chain as an immunologic biomarker of prognosis and chemotherapy response in solid tumors. Oncoimmunology. https://doi.org/10.4161/onci.21653
Chen Z, Gerhold-Ay A, Gebhard S et al (2012) Immunoglobulin kappa C predicts overall survival in node-negative breast cancer. PLoS ONE. https://doi.org/10.1371/journal.pone.0044741
Whiteside TL, Ferrone S (2012) For breast cancer prognosis, immunoglobulinkappa chain surfaces to the top. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-12-0566
Teillaud JL, Dieu-Nosjean MC (2017) Tertiary lymphoid structures: An anti-tumor school for adaptive immune cells and an antibody factory to fight cancer? Front Immunol. https://doi.org/10.3389/fimmu.2017.00830
Erdag G, Schaefer JT, Smolkin ME et al (2012) Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-11-3218
Lohr M, Edlund K, Botling J et al (2013) The prognostic relevance of tumour-infiltrating plasma cells and immunoglobulin kappa C indicates an important role of the humoral immune response in non-small cell lung cancer. Cancer Lett. https://doi.org/10.1016/j.canlet.2013.01.036
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Seow, D.Y.B., Yeong, J.P.S., Lim, J.X. et al. Tertiary lymphoid structures and associated plasma cells play an important role in the biology of triple-negative breast cancers. Breast Cancer Res Treat 180, 369–377 (2020). https://doi.org/10.1007/s10549-020-05548-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05548-y