Abstract
Objective
Extracellular vesicles (EVs) are subcellular signalosomes. Although characteristic EV production is associated with numerous physiological and pathological conditions, the effect of blood-derived EVs on bone homeostasis is unknown. Herein we evaluated the role of circulating EVs on human osteoclastogenesis.
Methods
Blood samples from healthy volunteers, rheumatoid arthritis (RA) and psoriatic arthritis (PsA) patients were collected. Size-based EV sub-fractions were isolated by gravity-driven filtration and differential centrifugation. To investigate the properties of EV samples, resistive pulse sensing technique, transmission electron microscopy, flow cytometry and western blot were performed. CD14+ monocytes were separated from PBMCs, and stimulated with recombinant human M-CSF, RANKL and blood-derived EV sub-fractions. After 7 days, the cells were fixed and stained for tartrate-resistant acid phosphatase and counted.
Results
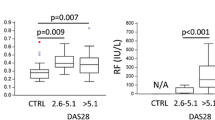
EVs isolated by size-based sub-fractions were characterized as either microvesicles or exosomes (EXO). Healthy (n = 11) and RA-derived (n = 12) EXOs profoundly inhibited osteoclast differentiation (70%, p < 0.01; 65%, p < 0.01, respectively). In contrast, PsA-derived (n = 10) EXOs had a stimulatory effect (75%, p < 0.05). In cross-treatment experiments where EXOs and CD14+ cells were interchanged between the three groups, only healthy (n = 5) and RA (n = 5)-derived EXOs inhibited (p < 0.01, respectively) the generation of osteoclasts in all groups, whereas PsA (n = 7)-derived EXOs were unable to mediate this effect.
Conclusions
Our data suggest that blood-derived EXOs are novel regulators of the human osteoclastogenesis and may offer discrete effector function in distinct inflammatory arthropathies.






Similar content being viewed by others
Abbreviations
- ACD-A:
-
Acid citrate dextrose-A
- ACR:
-
American College of Rheumatology
- ANOVA:
-
Analysis of variance
- ATCC:
-
American Type Culture Collection
- CALCR:
-
Calcitonin receptor
- CD14:
-
Cluster of differentiation 14
- C-FOS:
-
Cellular oncogene Fos
- C-FMS:
-
Macrophage colony-stimulating factor receptor
- CTSK:
-
Catepsin K
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- DAS:
-
Disease activity score
- DC STAMP:
-
Transmembrane 7 superfamily member 4
- EULAR:
-
European League Against Rheumatism
- EV:
-
Extracellular vesicle
- EXO:
-
Exosome
- FACS:
-
Fluorescence-activated cell sorting
- FBS:
-
Fetal bovine serum
- FITC:
-
Fluorescein isothiocyanate
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- IgG:
-
Immunoglobulin G
- ISEV:
-
International Society for Extracellular Vesicles
- LPS:
-
Lipopolysaccharide
- M-CSF:
-
Macrophage colony-stimulating factor
- miRNA:
-
Micro-ribonucleicacid
- MV:
-
Microvesicle
- NFATc1:
-
Nuclear factor of activated T cells cytoplasmic 1
- OC:
-
Osteoclast
- OSCAR:
-
Osteoclast-associated immunglobulin-like receptor
- PBMC:
-
Peripheral blood mononuclear cell
- PBS:
-
Phosphate-buffered saline
- PCR:
-
Polymerase chain reaction
- RANK:
-
Receptor activator of nuclear factor kappa B
- RANKL:
-
RANK ligand
- RPM:
-
Revolution per minute
- RPMI:
-
Roswell Park Memorial Institute medium
- rSPA:
-
Recombinant Staphylococcal Protein A
- RT:
-
Room temperature
- SEC:
-
Size-exclusion chromatography
- SIC:
-
Soluble immune complex
- SLAP:
-
Src-like adaptor protein
- TEM:
-
Transmission electron microscopy
- TRAP:
-
Tartrate-resistant acid phosphatase
References
Keller S, Ridinger J, Rupp AK, Janssen JW, Altevogt P (2011) Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med 9:86. doi:10.1186/1479-5876-9-86
Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Thery C (2016) Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA 113(8):E968–E977. doi:10.1073/pnas.1521230113
Lee EY, Choi DY, Kim DK, Kim JW, Park JO, Kim S, Kim SH, Desiderio DM, Kim YK, Kim KP, Gho YS (2009) Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics 9(24):5425–5436. doi:10.1002/pmic.200900338
Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Thery C (2014) Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 3:26913
Thery C, Zitvogel L, Amigorena S (2002) Exosomes: composition, biogenesis and function. Nat Rev Immunol 2(8):569–579. doi:10.1038/nri855
Szabo GT, Tarr B, Paloczi K, Eder K, Lajko E, Kittel A, Toth S, Gyorgy B, Pasztoi M, Nemeth A, Osteikoetxea X, Pallinger E, Falus A, Szabo-Taylor K, Buzas EI (2014) Critical role of extracellular vesicles in modulating the cellular effects of cytokines. Cell Mol Life Sci 71(20):4055–4067. doi:10.1007/s00018-014-1618-z
Sellam J, Proulle V, Jungel A, Ittah M, Miceli Richard C, Gottenberg JE, Toti F, Benessiano J, Gay S, Freyssinet JM, Mariette X (2009) Increased levels of circulating microparticles in primary Sjogren’s syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res Ther 11(5):R156. doi:10.1186/ar2833
Buzas EI, Gyorgy B, Nagy G, Falus A, Gay S (2014) Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol. doi:10.1038/nrrheum.2014.19
Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C (2005) Exosomal-like vesicles are present in human blood plasma. Int Immunol 17(7):879–887. doi:10.1093/intimm/dxh267
Cloutier N, Pare A, Farndale RW, Schumacher HR, Nigrovic PA, Lacroix S, Boilard E (2012) Platelets can enhance vascular permeability. Blood 120(6):1334–1343. doi:10.1182/blood-2012-02-413047
Skriner K, Adolph K, Jungblut PR, Burmester GR (2006) Association of citrullinated proteins with synovial exosomes. Arthritis Rheum 54(12):3809–3814. doi:10.1002/art.22276
Lo Cicero A, Majkowska I, Nagase H, Di Liegro I, Troeberg L (2012) Microvesicles shed by oligodendroglioma cells and rheumatoid synovial fibroblasts contain aggrecanase activity. Matrix Biol 31(4):229–233. doi:10.1016/j.matbio.2012.02.005
Cheung KL, Jarrett R, Subramaniam S, Salimi M, Gutowska-Owsiak D, Chen YL, Hardman C, Xue L, Cerundolo V, Ogg G (2016) Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J Exp Med 213(11):2399–2412. doi:10.1084/jem.20160258
Lee JY, Park JK, Lee EY, Lee EB, Song YW (2016) Circulating exosomes from patients with systemic lupus erythematosus induce an proinflammatory immune response. Arthritis Res Ther 18(1):264. doi:10.1186/s13075-016-1159-y
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JMW, Hobbs K, Huizinga TWJ, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Ménard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovský J, Wolfe F, Hawker G (2010) 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 62(9):2569–2581. doi:10.1002/art.27584
Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H (2006) Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 54(8):2665–2673. doi:10.1002/art.21972
Mc Ardle A, Flatley B, Pennington SR, FitzGerald O (2015) Early biomarkers of joint damage in rheumatoid and psoriatic arthritis. Arthritis Res Ther 17:141. doi:10.1186/s13075-015-0652-z
Takayanagi H (2009) Osteoimmunology and the effects of the immune system on bone. Nat Rev Rheumatol 5(12):667–676. doi:10.1038/nrrheum.2009.217
Harre U, Georgess D, Bang H, Bozec A, Axmann R, Ossipova E, Jakobsson PJ, Baum W, Nimmerjahn F, Szarka E, Sarmay G, Krumbholz G, Neumann E, Toes R, Scherer HU, Catrina AI, Klareskog L, Jurdic P, Schett G (2012) Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Invest 122(5):1791–1802. doi:10.1172/JCI60975
Oliveira MC, Di Ceglie I, Arntz OJ, van den Berg WB, van den Hoogen FH, Ferreira AV, van Lent PL, van de Loo FA (2016) Milk-derived nanoparticle fraction promotes the formation of small osteoclasts but reduces bone resorption. J Cell Physiol. doi:10.1002/jcp.25414
Deng L, Wang Y, Peng Y, Wu Y, Ding Y, Jiang Y, Shen Z, Fu Q (2015) Osteoblast-derived microvesicles: a novel mechanism for communication between osteoblasts and osteoclasts. Bone 79:37–42. doi:10.1016/j.bone.2015.05.022
Huynh N, VonMoss L, Smith D, Rahman I, Felemban MF, Zuo J, Rody WJ Jr, McHugh KP, Holliday LS (2016) Characterization of regulatory extracellular vesicles from osteoclasts. J Dent Res. doi:10.1177/0022034516633189
Li D, Liu J, Guo B, Liang C, Dang L, Lu C, He X, Cheung HY, Xu L, He B, Liu B, Shaikh AB, Li F, Wang L, Yang Z, Au DW, Peng S, Zhang Z, Zhang BT, Pan X, Qian A, Shang P, Xiao L, Jiang B, Wong CK, Xu J, Bian Z, Liang Z, Guo DA, Zhu H, Tan W, Lu A, Zhang G (2016) Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat Commun 7:10872. doi:10.1038/ncomms10872
Gyori D, Csete D, Benko S, Kulkarni S, Mandl P, Dobo-Nagy C, Vanhaesebroeck B, Stephens L, Hawkins PT, Mocsai A (2014) The phosphoinositide 3-kinase isoform PI3Kbeta regulates osteoclast-mediated bone resorption in humans and mice. Arthritis Rheumatol 66(8):2210–2221. doi:10.1002/art.38660
Gyorgy B, Paloczi K, Kovacs A, Barabas E, Beko G, Varnai K, Pallinger E, Szabo-Taylor K, Szabo TG, Kiss AA, Falus A, Buzas EI (2014) Improved circulating microparticle analysis in acid-citrate dextrose (ACD) anticoagulant tube. Thromb Res 133(2):285–292. doi:10.1016/j.thromres.2013.11.010
Gyorgy B, Szabo TG, Turiak L, Wright M, Herczeg P, Ledeczi Z, Kittel A, Polgar A, Toth K, Derfalvi B, Zelenak G, Borocz I, Carr B, Nagy G, Vekey K, Gay S, Falus A, Buzas EI (2012) Improved flow cytometric assessment reveals distinct microvesicle (cell-derived microparticle) signatures in joint diseases. PLoS One 7(11):e49726. doi:10.1371/journal.pone.0049726
Baranyai T, Herczeg K, Onodi Z, Voszka I, Modos K, Marton N, Nagy G, Mager I, Wood MJ, El Andaloussi S, Palinkas Z, Kumar V, Nagy P, Kittel A, Buzas EI, Ferdinandy P, Giricz Z (2015) Isolation of exosomes from blood plasma: qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS One 10(12):e0145686. doi:10.1371/journal.pone.0145686
Osteikoetxea X, Sodar B, Nemeth A, Szabo-Taylor K, Paloczi K, Vukman KV, Tamasi V, Balogh A, Kittel A, Pallinger E, Buzas EI (2015) Differential detergent sensitivity of extracellular vesicle subpopulations. Org Biomol Chem 13(38):9775–9782. doi:10.1039/c5ob01451d
Sodar BW, Kittel A, Paloczi K, Vukman KV, Osteikoetxea X, Szabo-Taylor K, Nemeth A, Sperlagh B, Baranyai T, Giricz Z, Wiener Z, Turiak L, Drahos L, Pallinger E, Vekey K, Ferdinandy P, Falus A, Buzas EI (2016) Low-density lipoprotein mimics blood plasma-derived exosomes and microvesicles during isolation and detection. Sci Rep 6:24316. doi:10.1038/srep24316
Gyorgy B, Modos K, Pallinger E, Paloczi K, Pasztoi M, Misjak P, Deli MA, Sipos A, Szalai A, Voszka I, Polgar A, Toth K, Csete M, Nagy G, Gay S, Falus A, Kittel A, Buzas EI (2011) Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood 117(4):e39–e48. doi:10.1182/blood-2010-09-307595
MacLellan LM, Montgomery J, Sugiyama F, Kitson SM, Thummler K, Silverman GJ, Beers SA, Nibbs RJ, McInnes IB, Goodyear CS (2011) Co-opting endogenous immunoglobulin for the regulation of inflammation and osteoclastogenesis in humans and mice. Arthritis Rheum 63(12):3897–3907. doi:10.1002/art.30629
Boing AN, van der Pol E, Grootemaat AE, Coumans FA, Sturk A, Nieuwland R (2014) Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles. doi:10.3402/jev.v3.23430
Ell B, Mercatali L, Ibrahim T, Campbell N, Schwarzenbach H, Pantel K, Amadori D, Kang Y (2013) Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer Cell 24(4):542–556. doi:10.1016/j.ccr.2013.09.008
Inder KL, Ruelcke JE, Petelin L, Moon H, Choi E, Rae J, Blumenthal A, Hutmacher D, Saunders NA, Stow JL, Parton RG, Hill MM (2014) Cavin-1/PTRF alters prostate cancer cell-derived extracellular vesicle content and internalization to attenuate extracellular vesicle-mediated osteoclastogenesis and osteoblast proliferation. J Extracell Vesicles. doi:10.3402/jev.v3.23784
Raimondi L, De Luca A, Amodio N, Manno M, Raccosta S, Taverna S, Bellavia D, Naselli F, Fontana S, Schillaci O, Giardino R, Fini M, Tassone P, Santoro A, De Leo G, Giavaresi G, Alessandro R (2015) Involvement of multiple myeloma cell-derived exosomes in osteoclast differentiation. Oncotarget 6(15):13772–13789. doi:10.18632/oncotarget.3830
Osteikoetxea X, Balogh A, Szabo-Taylor K, Nemeth A, Szabo TG, Paloczi K, Sodar B, Kittel A, Gyorgy B, Pallinger E, Matko J, Buzas EI (2015) Improved characterization of EV preparations based on protein to lipid ratio and lipid properties. PLoS One 10(3):e0121184. doi:10.1371/journal.pone.0121184
Artoni A, Merati G, Padovan L, Scalambrino E, Chantarangkul V, Tripodi A (2012) Residual platelets are the main determinants of microparticles count in frozen-thawed plasma. Thromb Res 130(3):561–562. doi:10.1016/j.thromres.2012.04.012
Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, Laszlo V, Pallinger E, Pap E, Kittel A, Nagy G, Falus A, Buzas EI (2011) Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci 68(16):2667–2688. doi:10.1007/s00018-011-0689-3
Willekens FL, Werre JM, Kruijt JK, Roerdinkholder-Stoelwinder B, Groenen-Dopp YA, van den Bos AG, Bosman GJ, van Berkel TJ (2005) Liver Kupffer cells rapidly remove red blood cell-derived vesicles from the circulation by scavenger receptors. Blood 105(5):2141–2145. doi:10.1182/blood-2004-04-1578
Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF, Zhou Q, Sui SF (2010) Cellular internalization of exosomes occurs through phagocytosis. Traffic 11(5):675–687. doi:10.1111/j.1600-0854.2010.01041.x
Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D (2012) Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 18(6):883–891. doi:10.1038/nm.2753
Szarka E, Babos F, Magyar A, Huber K, Szittner Z, Papp K, Prechl J, Pozsgay J, Neer Z, Adori M, Nagy G, Rojkovich B, Gati T, Kelemen J, Baka Z, Brozik M, Pazar B, Poor G, Hudecz F, Sarmay G (2014) Recognition of new citrulline-containing peptide epitopes by autoantibodies produced in vivo and in vitro by B cells of rheumatoid arthritis patients. Immunology 141(2):181–191. doi:10.1111/imm.12175
Knijff-Dutmer EA, Koerts J, Nieuwland R, Kalsbeek-Batenburg EM, van de Laar MA (2002) Elevated levels of platelet microparticles are associated with disease activity in rheumatoid arthritis. Arthritis Rheum 46(6):1498–1503. doi:10.1002/art.10312
Veale DJ, Fearon U (2015) What makes psoriatic and rheumatoid arthritis so different? RMD Open 1(1):e000025. doi:10.1136/rmdopen-2014-000025
Acknowledgements
This work was supported by the Hungarian Scientific Research Fund OTKA-NN111023, OTKA-NKFIH #11958; MEDINPROT and BMBS COST Action BM1202 ME HAD. Funding was provided by National Heart Program (Grant Nos. OTKA 120237, NKFIA, and KP-16-1-2016-0017).
Author information
Authors and Affiliations
Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Nagy had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
18_2017_2535_MOESM1_ESM.tif
Supplementary Figure 1 Flow cytometry gate determination for ‘MV’ analysis. A, The experiment was carried out using MegaMix Beads (BioCytex, Marseille, France) and was optimized with 1 μm Silica Beads Fluo-Green (Kisker, Steinfurt, Germany). Logarithmic FSC, SSC scales were used during the measurements to visualize the ‘MVs’. ‘MVs’ are EVs of 100–1000 nm diameter. B, C, Decreased number of Annexin V positive events were detected in the ‘MV gate’ after detergent treatment. (TIFF 3711 kb)
18_2017_2535_MOESM2_ESM.tif
Supplementary Figure 2 The effect of different concentrations of SICs on the OC differentiation. SICs were generated freshly and used in different concentrations: 0.25X; 0.5X; 1X and 2X SIC treatment means that 0.25; 0.5; 1 and 2 µL of SIC samples were added to 100 µL media. The graph represents the fold increase in the number TRAP-positive cells with ≥ 3 nuclei. The values mark the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. (TIFF 2480 kb)
18_2017_2535_MOESM3_ESM.tif
Supplementary Figure 3 The effect of size exclusion chromatography purified EXO samples on human in vitro osteoclastogenesis. Platelet free plasma samples of healthy donors (n = 2) were filtrated with 0.8 µm nanopore membranes and purified with qEV Size Exclusion Columns (Izon Science, Christchurch, New Zealand) according to manufacturer’s instructions. Then the samples were centrifuged with 100 000g, resuspended in 1*PBS and used to treat differentiating OC samples as in the previously described experiments. The graph represents the fold increase in the number of OCs (TRAP-positive cells with ≥ 3 nuclei). The values represent the mean ± SEM. *p < 0.05 ‘EXO’ refers to small EVs of approximately 100 nm diameter. (TIFF 735 kb)
18_2017_2535_MOESM4_ESM.jpg
Supplementary Figure 4 OPG, RANK, RANKL expression of EVs. EXO samples, conjugated to aldehyde/sulfate latex beads were studied by flow cytometry. Differential detergent lysis was used to study the MVs. The values represent the mean ± SEM. *p < 0.05. (JPEG 189 kb)
18_2017_2535_MOESM5_ESM.tif
Supplementary Figure 5 The detection of various CD markers on EV samples. EXO samples, conjugated to aldehyde/sulfate latex beads were studied by flow cytometry. Differential detergent lysis was used to identify the MVs. The values represent the mean ± SEM. *p < 0.05. (TIFF 13634 kb)
Rights and permissions
About this article
Cite this article
Marton, N., Kovács, O.T., Baricza, E. et al. Extracellular vesicles regulate the human osteoclastogenesis: divergent roles in discrete inflammatory arthropathies. Cell. Mol. Life Sci. 74, 3599–3611 (2017). https://doi.org/10.1007/s00018-017-2535-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2535-8