Abstract
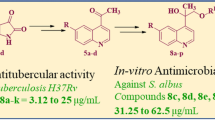
A series of secondary amines (4–19) containing 2-chloroquinoline as lipophilic domain have been synthesized based on the structural requirements essential for allylamine/benzylamine antimycotics by nucleophilic substitution reaction of 3-chloromethyl-2-chloroquinoline 3 with various aliphatic and aromatic amines in absolute ethanol in the presence of triethylamine. Some N-methyl derivatives (20–25) were also synthesized by N-methylation using (CH3)2SO4/NaH. The structures of newly synthesized compounds were established by the combined use of IR, 1H-NMR, 13C-NMR, and Mass spectra. Compounds 4–25 were screened in vitro at conc. of 200 g/ml for their antifungal activity against Aspergillus niger MTCC 281, Aspergillus flavus MTCC 277, Monascus purpureus MTCC 369, and Penicillium citrinum NCIM 768 by cup-plate method using Terbinafine as a references drug. Among the secondary amines, compounds 4, 5, 8, 10, 14, and 16 exhibited potential antifungal activity and their corresponding N-methyl (20–25) derivatives also showed further increase in antifungal activity against fungal strain Aspergillus niger MTCC 281, Aspergillus flavus MTCC 277. Compounds 3-chloro-N-[(2-chloroquinolin-3-yl)methyl]-4-fluoro-N-methylaniline (24) and 3,4-dichloro-N-[(2-chloroquinolin-3-yl)methyl]-N-methylaniline (25) were the most potent derivatives of the series.


Similar content being viewed by others
References
Acharya BN, Thavaselvam D, Kaushik MP (2008) Synthesis and antimalarial evaluation of novel pyridine quinoline hybrids. Med Chem Res 17(8):487–494
Arnoldi A, Merlini L (1990) Lipophilicity-antifungal activity relationship for some isoflavonoid phytoalexins. J Agric Food Chem 38:834–838
Cruickshank R, Duguid JP, Marion BP, Swain RHA (1975) Medical microbiology, vol 2, 12th edn. Churchill Livingstone, London
Delgado JN, Remers WA (2005) Wilson and Gisvold’s Textbook of organic medicinal and pharmaceutical chemistry, 10th edn. Lippincott Williams & Wilkins, Philadelphia
Favre B, Ryder NS (1997) Differential inhibition of fungal and mammalian squalene epoxidases by Benzylamine SDZ SBA 586 in comparison with allylamine Terbinafine. Arch Biochem Biophys 340(2):265–269
Meth-Cohn O, Narine B, Tarnowski B (1981) A versatile new synthesis of quinoline and related fused pyridines. Part-5. The synthesis of 2-chloroquinoline-3-carboxaldehyde. J Chem Soc Perkin Trans I 5:1520–1530
Munawar MA, Azad M, Athar M, Groundwater PW (2008) Synthesis and antimicrobial activity of quinoline-based-2-pyrazoline. Chem Lett 62(3):288–293
Musiol R, Jampilek J, Buchta V, Silva L, Niedbala H, Podeszwa B, Palka A, Majerz-Manieck K, Oleksyn B, Polanski J (2006) Antifungal properties of new series of quinoline derivatives. Bioorg Med Chem 14(10):3592–3598
Nussbaumer P, Petranyi G, Stutz A (1991) Synthesis and structure-activity relationship of benzo[b]thienylallylamine. J Med Chem 34:65–73
Nussbaumer P, Leitner I, Stutz A (1994) Synthesis and structure- activity relationship of the novel homopropargylamine antimycotics. J Med Chem 37:610–615
Prisyazhnyuk PV, Patratii VK, Prodanchuk NG, Tashchuk KG, Fedoryak SD (1984) Synthesis and antimicrobial properties of some quinoline derivatives. Pharm Chem J 18(4):258–262
Rizk OH, Mahran MA, El-Khawass SM, Shams El-Dine SA, Ibrahim EA (2005) Synthesis of some new antimicrobial thiadiazolyl and oxadiazolyl quinoline derivatives. Med Chem Res 14(5):260–273
Strigáčová J, Hudecová D, Varečka L, Lásiková A, Végh D (2000) Some biological properties of new quinoline-4-carboxylic acid and quinoline-4-carboxamide derivatives. Folia Microbiol 45(4):305–309
Stutz A, Georgopoulos A, Granitzer W, Petranyi G, Berney D (1986) Synthesis nd structure-activity relationship of Naftifine-related allylmine antimycotics. J Med Chem 29:112–125
Varma RS, Khan ZK, Singh AP (1998) Antifungal agents: past present future prospects. National Academy of Chemistry and Biology, Lucknow
Acknowledgments
The author (SK) is thankful to the University Grant Commission (UGC), New Delhi, India, for the award of UGC-JRF. The authors are also thankful to Department of pharmaceutical chemistry, faculty of pharmacy, Jamia Hamdard for providing necessary facility and CDRI, Lucknow, India for recording FAB-MS spectral data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, S., Bawa, S., Drabu, S. et al. Design and synthesis of 2-chloroquinoline derivatives as non-azoles antimycotic agents. Med Chem Res 20, 1340–1348 (2011). https://doi.org/10.1007/s00044-010-9463-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-010-9463-6