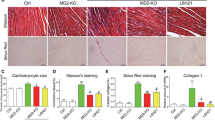
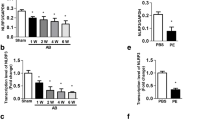
Abstract
Cardiac hypertrophy is a complex pathological process, and the molecular mechanisms underlying hypertrophic remodeling have not been clearly elucidated. Leukocyte immunoglobulin-like receptor B4 (lilrb4) is an inhibitory transmembrane protein that is necessary for the regulation of various cellular signaling pathways. To investigate whether lilrb4 plays a role in cardiac hypertrophy, we performed aortic banding in lilrb4 knockout mice, lilrb4 cardiac-specific transgenic mice, and their wild-type littermates. Cardiac hypertrophy was evaluated by echocardiographic, hemodynamic, pathological, and molecular analyses. We found that lilrb4 was expressed both in myocardial tissue and on cultured cardiomyocytes under basal conditions, but the expression was obviously decreased in mouse hearts following aortic banding and in cardiomyocytes treated with angiotensin II. Lilrb4 disruption aggravated cardiac hypertrophy, fibrosis, and dysfunction in response to pressure overload. Conversely, the cardiac overexpression of lilrb4 led to the opposite effects. Moreover, lilrb4 overexpression inhibited angiotensin II-induced cardiomyocyte hypertrophy in vitro. Mechanistically, we determined that the cardioprotective effect of lilrb4 was mediated through an interaction with SHP-2, the preservation of phosphorylated SHP-2, and the inhibition of the NF-κB pathway. In addition, SHP-2 knockdown in cardiomyocytes eliminated the inhibitory effects of lilrb4 on angiotensin II-induced hypertrophy and NF-κB activation. Our results suggest that lilrb4 protects against pathological cardiac hypertrophy via the SHP-2-dependent inhibition of the NF-κB pathway and may act as a potential therapeutic target for cardiac hypertrophy.
Key messages
-
Lilrb4 expression is decreased by hypertrophic stimuli.
-
Lilrb4 protects against pathological cardiac hypertrophy.
-
Lilrb4 interacts with SHP-2 and inhibits NF-κB pathway.








Similar content being viewed by others
References
Zhou H, Li N, Yuan Y, Jin YG, Guo H, Deng W, Tang QZ (2018) Activating transcription factor 3 in cardiovascular diseases: a potential therapeutic target. Basic Res Cardiol 113(5):37
Rautaharju PM, Soliman EZ (2014) Electrocardiographic left ventricular hypertrophy and the risk of adverse cardiovascular events: a critical appraisal. J Electrocardiol 47(5):649–654
Wu QQ, Xiao Y, Yuan Y, Ma ZG, Liao HH, Liu C, Zhu JX, Yang Z, Deng W, Tang QZ (2017) Mechanisms contributing to cardiac remodelling. Clin Sci (Lond) 131(18):2319–2345
Cella M, Dohring C, Samaridis J, Dessing M, Brockhaus M, Lanzavecchia A, Colonna M (1997) A novel inhibitory receptor (ILT3) expressed on monocytes, macrophages, and dendritic cells involved in antigen processing. J Exp Med 185(10):1743–1751
Bryceson YT, Torgersen KM, Inngjerdingen M, Berg SF, Hoelsbrekken SE, Fossum S, Dissen E (2005) The rat orthologue to the inhibitory receptor gp49B is expressed by neutrophils and monocytes, but not by NK cells or mast cells. Eur J Immunol 35(4):1230–1239
Lee KH, Ono M, Inui M, Yuasa T, Takai T (2000) Stimulatory function of gp49A, a murine Ig-like receptor, in rat basophilic leukemia cells. J Immunol 165(9):4970–4977
Katz HR (2007) Inhibition of pathologic inflammation by leukocyte Ig-like receptor B4 and related inhibitory receptors. Immunol Rev 217:222–230
Kuroiwa A, Yamashita Y, Inui M, Yuasa T, Ono M, Nagabukuro A, Matsuda Y, Takai T (1998) Association of tyrosine phosphatases SHP-1 and SHP-2, inositol 5-phosphatase SHIP with gp49B1, and chromosomal assignment of the gene. J Biol Chem 273(2):1070–1074
Lu HK, Rentero C, Raftery MJ, Borges L, Bryant K, Tedla N (2009) Leukocyte Ig-like receptor B4 (LILRB4) is a potent inhibitor of FcgammaRI-mediated monocyte activation via dephosphorylation of multiple kinases. J Biol Chem 284(50):34839–34848
Cortesini R, Suciu-Foca N (2006) ILT3+ ILT4+ tolerogenic endothelial cells in transplantation. Transplantation 82(1 Suppl):S30–S32
Jensen MA, Yanowitch RN, Reder AT, White DM, Arnason BG (2010) Immunoglobulin-like transcript 3, an inhibitor of T cell activation, is reduced on blood monocytes during multiple sclerosis relapses and is induced by interferon beta-1b. Mult Scler 16(1):30–38
Deng M, Gui X, Kim J, Xie L, Chen W, Li Z, He L, Chen Y, Chen H, Luo W et al (2018) LILRB4 signalling in leukaemia cells mediates T cell suppression and tumour infiltration. Nature 562(7728):605–609
Lu Y, Jiang Z, Dai H, Miao R, Shu J, Gu H, Liu X, Huang Z, Yang G, Chen AF et al (2018) Hepatic leukocyte immunoglobulin-like receptor B4 (LILRB4) attenuates nonalcoholic fatty liver disease via SHP1-TRAF6 pathway. Hepatology 67(4):1303–1319
Jiang Z, Qin JJ, Zhang Y, Cheng WL, Ji YX, Gong FH, Zhu XY, She ZG, Huang Z, Li H (2017) LILRB4 deficiency aggravates the development of atherosclerosis and plaque instability by increasing the macrophage inflammatory response via NF-kappaB signaling. Clin Sci (Lond) 131(17):2275–2288. https://doi.org/10.1042/CS20170198
Jiang DS, Wei X, Zhang XF, Liu Y, Zhang Y, Chen K, Gao L, Zhou H, Zhu XH, Liu PP et al (2014) IRF8 suppresses pathological cardiac remodelling by inhibiting calcineurin signalling. Nat Commun 5:3303
Jiang DS, Liu Y, Zhou H, Zhang Y, Zhang XD, Zhang XF, Chen K, Gao L, Peng J, Gong H et al (2014) Interferon regulatory factor 7 functions as a novel negative regulator of pathological cardiac hypertrophy. Hypertension 63(4):713–722
Zhou H, Bian ZY, Zong J, Deng W, Yan L, Shen DF, Guo H, Dai J, Yuan Y, Zhang R et al (2012) Stem cell antigen 1 protects against cardiac hypertrophy and fibrosis after pressure overload. Hypertension 60(3):802–809
Marin TM, Clemente CF, Santos AM, Picardi PK, Pascoal VD, Lopes-Cendes I, Saad MJ, Franchini KG (2008) Shp2 negatively regulates growth in cardiomyocytes by controlling focal adhesion kinase/Src and mTOR pathways. Circ Res 103(8):813–824
Vlad G, Chang CC, Colovai AI, Vasilescu ER, Cortesini R, Suciu-Foca N (2010) Membrane and soluble ILT3 are critical to the generation of T suppressor cells and induction of immunological tolerance. Int Rev Immunol 29(2):119–132
Penna G, Roncari A, Amuchastegui S, Daniel KC, Berti E, Colonna M, Adorini L (2005) Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood 106(10):3490–3497
Buckland M, Lombardi G (2009) Aspirin and the induction of tolerance by dendritic cells. Handb Exp Pharmacol 188:197–213
Tilstra JS, Clauson CL, Niedernhofer LJ, Robbins PD (2011) NF-kappaB in aging and disease. Aging Dis 2(6):449–465
Christian F, Smith EL, Carmody RJ (2016) The regulation of NF-kappaB subunits by phosphorylation. Cells 5(1). https://doi.org/10.3390/cells5010012
Purcell NH, Tang G, Yu C, Mercurio F, DiDonato JA, Lin A (2001) Activation of NF-kappa B is required for hypertrophic growth of primary rat neonatal ventricular cardiomyocytes. Proc Natl Acad Sci U S A 98(12):6668–6673
Liu Q, Chen Y, Auger-Messier M, Molkentin JD (2012) Interaction between NFkappaB and NFAT coordinates cardiac hypertrophy and pathological remodeling. Circ Res 110(8):1077–1086
Gupta S, Young D, Maitra RK, Gupta A, Popovic ZB, Yong SL, Mahajan A, Wang Q, Sen S (2008) Prevention of cardiac hypertrophy and heart failure by silencing of NF-kappaB. J Mol Biol 375(3):637–649
Chang CC, Liu Z, Vlad G, Qin H, Qiao X, Mancini DM, Marboe CC, Cortesini R, Suciu-Foca N (2009) Ig-like transcript 3 regulates expression of proinflammatory cytokines and migration of activated T cells. J Immunol 182(9):5208–5216
Qiu T, Zhou J, Wang T, Chen Z, Ma X, Zhang L, Zou J (2019) Leukocyte immunoglobulin-like receptor B4 deficiency exacerbates acute lung injury via NF-kappaB signaling in bone marrow-derived macrophages. Biosci Rep 39(6). https://doi.org/10.1042/BSR20181888
Zhou H, Shen DF, Bian ZY, Zong J, Deng W, Zhang Y, Guo YY, Li H, Tang QZ (2011) Activating transcription factor 3 deficiency promotes cardiac hypertrophy, dysfunction, and fibrosis induced by pressure overload. PLoS One 6(10):e26744
Li Q, Wei G, Tao T (2019) Leukocyte immunoglobulin-like receptor B4 (LILRB4) negatively mediates the pathological cardiac hypertrophy by suppressing fibrosis, inflammation and apoptosis via the activation of NF-kappaB signaling. Biochem Biophys Res Commun 509(1):16–23
Chong ZZ, Maiese K (2007) The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol Histopathol 22(11):1251–1267
Tajan M, de Rocca SA, Valet P, Edouard T, Yart A (2015) SHP2 sails from physiology to pathology. Eur J Med Genet 58(10):509–525
Kontaridis MI, Yang W, Bence KK, Cullen D, Wang B, Bodyak N, Ke Q, Hinek A, Kang PM, Liao R, Neel BG (2008) Deletion of Ptpn11 (Shp2) in cardiomyocytes causes dilated cardiomyopathy via effects on the extracellular signal-regulated kinase/mitogen-activated protein kinase and RhoA signaling pathways. Circulation 117(11):1423–1435
Coulombe G, Leblanc C, Cagnol S, Maloum F, Lemieux E, Perreault N, Feng GS, Boudreau F, Rivard N (2013) Epithelial tyrosine phosphatase SHP-2 protects against intestinal inflammation in mice. Mol Cell Biol 33(11):2275–2284
Heun Y, Pircher J, Czermak T, Bluem P, Hupel G, Bohmer M, Kraemer BF, Pogoda K, Pfeifer A, Woernle M et al (2019) Inactivation of the tyrosine phosphatase SHP-2 drives vascular dysfunction in sepsis. EBioMedicine 42:120–132
You M, Flick LM, Yu D, Feng GS (2001) Modulation of the nuclear factor kappa B pathway by Shp-2 tyrosine phosphatase in mediating the induction of interleukin (IL)-6 by IL-1 or tumor necrosis factor. J Exp Med 193(1):101–110
Kapoor GS, Zhan Y, Johnson GR, O'Rourke DM (2004) Distinct domains in the SHP-2 phosphatase differentially regulate epidermal growth factor receptor/NF-kappaB activation through Gab1 in glioblastoma cells. Mol Cell Biol 24(2):823–836
Acknowledgments
We thank Dr. Toshiyuki Takai (Tohoku University) for generously providing the lilrb4 knockout mice.
Funding
This work was supported by the National Natural Science Foundation of China (81770399, 81300070, 81530012 and 81700218), the Fundamental Research Funds for the Central Universities of China (2042018kf0121 and 2042018kf1032), National Key R&D Program of China (2018YFC1311300), Development Center for Medical Science and Technology National Health and Family Planning Commission of the People’s Republic of China (The prevention and control project of cardiovascular disease, 2016ZX-008-01), the National Natural Science Foundation of Hubei Province (2017CFB320).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, H., Li, N., Yuan, Y. et al. Leukocyte immunoglobulin-like receptor B4 protects against cardiac hypertrophy via SHP-2-dependent inhibition of the NF-κB pathway. J Mol Med 98, 691–705 (2020). https://doi.org/10.1007/s00109-020-01896-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-020-01896-w