Abstract
The antidiabetic drug metformin (MF) exhibits redox-modulating effects in various pathologies associated with oxidative stress and mitigates ionizing radiation-induced toxicity, but the underlying mechanisms remain to be elucidated. Thus, we studied some radiomitigatory effects of MF and explored the possible mechanisms behind them. Highly sensitive luminescence methods and non-competitive enzyme-linked immunosorbent assay (ELISA) were used in in vitro studies, and in vivo the damage to bone marrow cells and its repair were assessed by the micronucleus test. In a solution, MF at concentrations exceeding 0.1 µM effectively intercepts •OH upon X-ray-irradiation, but does not react directly with H2O2. MF accelerates the decomposition of H2O2 catalyzed by copper ions. MF does not affect the radiation-induced formation of H2O2 in the solution of bovine gamma-globulin (BGG), but has a modulating effect on the generation of H2O2 in the solution of bovine serum albumin (BSA). MF at 0.05–1 mM decreases the radiation-induced formation of 8-oxoguanine in a DNA solution depending on the concentration of MF with a maximum at 0.25 mM. MF at doses of 3 mg/kg body weight (bw) and 30 mg/kg bw administered to mice after irradiation, but not before irradiation, reduces the frequency of micronucleus formation in polychromatophilic erythrocytes of mouse red bone marrow. Our work has shown that the radiomitigatory properties of MF are mediated by antioxidant mechanisms of action, possibly including its ability to chelate polyvalent metal ions.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The study of new promising radiomitigators (Obrador et al. 2020) and radiotherapeutic agents, possible mechanisms of their action is an active area in current research. Ionizing irradiation of the organism induces excessive production of reactive oxygen species (ROS) and oxidative stress (OS). Exposure to ionizing radiation (IR) disturbs the redox homeostasis, damages proteins, cell membranes, nuclear and mitochondrial DNA, destroys a number of biological processes in cells and tissues of the body. Radiomitigators are applied to compensate for the formation and to repair damage caused by IR (Obrador et al. 2020). Although many chemical compounds are being investigated as protective agents, their use for medical purposes to eliminate the harmful effects of radiation is problematic given the lack of evidence on their long-term effectiveness and safety. To overcome this obstacle, we resorted to an alternative approach and searched radioprotective drugs among widely used conventional pharmaceuticals, because existing “older” drugs repositioned for new indications have well-known safety and pharmacologic profiles, and their therapeutic modalities for clinical applications are thoroughly investigated.
MF (1,1-dimethylbiguanidine hydrochloride) is the first-choice drug for the treatment of type 2 diabetes mellitus used since the late 1950s (DeFronzo and Goodman 1995). Recently, it has found use in radiation therapy of tumors and other pathological conditions (Piskovatska et al. 2020). MF protects only normal cells without preventing damage to cancer cells (Najafi et al. 2018) and also shows synergism with some chemotherapeutic agents (Honjo et al. 2014; Koritzinsky 2015). Beyond its hypoglycemic antidiabetic activity, MF reveals pleiotropic effects (Pietrocola and Kroemer 2017), causing a decrease in morbidity and mortality rates from cardiovascular diseases (Han et al. 2019; Schernthaner et al. 2022) and reduction in the risk of developing various oncological diseases (Pernicova and Korbonits 2014; Cheng et al. 2022). MF exhibits geroprotective and anticarcinogenic effects (Martin-Castillo et al. 2010; Chen et al. 2012; Campbell et al. 2017), can inhibit cellular ROS production and stimulate DNA damage responses (DeFronzo and Goodman 1995), thus reducing cancer risk. MF diminishes the risk of mortality in patients infected with SARS-CoV-2 virus (Bramante et al. 2021). MF acts as an inhibitor of complex I in mitochondria (Owen et al. 2000), which in turn leads to an AMP protein kinase (AMPK)-dependent (Hawley et al. 2010) and AMPK-independent (Kalender et al. 2010) cell response. MF is able to modulate the cellular metabolism and production of mitochondrial ROS by activation of AMPK (Toyama et al. 2016). MF inhibits tumor growth via affecting its downstream targets (including p53/p63/p73) and regulating signaling pathways (mTOR, NF-κB, and others) (Yi et al. 2019).
In general, MF affects the redox homeostasis of the body and has an impact on diseases associated with OS. Redox stress leads to genetic instability, an increase in the rate of mutations, loss of proteostasis, a decrease in life expectancy and to various diseases, including malignant tumors. There are few reports in the literature that MF is a potential versatile radiation modulator and radioprotector/radiomitigator (Miller et al. 2014; Abdullaev et al. 2018; Mortezaee et al. 2019; Yahyapour et al. 2019; Tajabadi et al. 2020; Wang et al. 2020). Thus, MF is a promising therapeutic agent capable of effectively ameliorating radiation toxicity. Although MF has long been used in medicine, mechanisms of its multiple effects are yet incompletely understood (Triggle et al. 2022). In this work, the antioxidant, genoprotective, and radiomitigatory properties of MF have been studied in vitro and in vivo upon X-ray-irradiation.
Materials and methods
Materials
The following reagents were used in the work: hydrogen peroxide (CAS: 7722-84-1), tris(hydroxymethyl) aminomethane (CAS: 77-86-1), 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS, CAS: 30931–67-0), bovine serum albumin (BSA, CAS: 9048-46-8), horseradish peroxidase (CAS: 9003-99-0), citric acid (CAS: 77-92-9), sodium citrate tribasic dihydrate (CAS: 6132-04-3), 4-iodophenol (CAS: 540-38-5), commercial high-polymer DNA from the salmon sperm (CAS: 9007-49-2), MF (1,1-dimethylbiguanide hydrochloride, CAS: 1115-70-4), sodium sulfate (Na2SO4, CAS: 7757-82-6), coumarin-3-carboxylic acid (CCA, CAS: 531-81-7), 7-OH-CCA (CAS: 779-27-1) (Sigma-Aldrich, USA), bovine gamma-globulin (BGG, CAS: 9007-83-4) (Serva, Germany), skimmed milk powder (Sampo, Russia), conjugate of secondary antibodies with horseradish peroxidase (Bio-Rad, USA), monoclonal antibodies to 8-oxoguanine (8-oxoG) (Bruskov et al. 1996), immersion oil (Cargill, USA), Giemsa-Romanowsky dye (Panreac, Spain), methanol (CAS: 67-56-1) (LaChema, France), luminol (CAS: 521-31-3) (AppliChem, Germany), hydrochloric acid (HCl, CAS: 7647-01-0) and copper sulfate (CuSO4 × 5H2O, CAS: 7758-99-8) (Reachim, Russia). Sodium chloride (NaCl, CAS: 7647-14-5), sodium phosphate mono- and dibasic (NaH2PO4 × 2H2O (CAS: 13472-35-0) and Na2HPO4 × 12H2O (CAS: 10039-32-4)) (Panreac, Spain) were used to prepare 1 mM phosphate buffer, pH 7.4 (PB), and phosphate buffered saline (PBS), pH 7.4 (PB + 150 mM NaCl). All reagents were used without further purification. Freshly prepared bidistilled water had pH of 5.8 and conductivity of 120 μS/m.
X-ray-irradiation
The animals and solutions were irradiated at the Common Use Center “Group of Radiation Sources” of the Institute of Cell Biophysics, Russian Academy of Sciences, using an RUT-15 therapeutical X-ray device (MosRentgen, Russia) at a dose rate of 1 Gy/min (200 kV, 20 mA, focal distance 37.5 cm). Filters (1 mm Cu/1 mm Al) of a half-value layer (HVL) of 1.6 mm Cu were used in the system. Instrumental dosimetry was carried out by the Center personnel using a dosimeter VA-J-18 type 27 006 (RFT (Rundfunk- und Fernmelde-Technik), Germany) with an ionization chamber as a radiation detector. Mice (5 animals per group) were irradiated at a dose of 1.5 Gy. Solutions were irradiated at doses of 5–15 Gy.
Measurement of hydrogen peroxide
The concentration of hydrogen peroxide in irradiated solutions of MF was measured by the method of enhanced chemiluminescence using a luminol–4-iodophenol–peroxidase system (Shtarkman et al. 2008). A counter for liquid scintillation Beta-1 (MedApparatura, Ukraine) operating in the mode of single photon counting was used as a highly sensitive chemiluminometer. The high sensitivity of this method allows registration of hydrogen peroxide at a concentration as low as 1 nM. The content of H2O2 was determined by using the calibration curves of chemiluminescence dependence on the known concentration of H2O2 in the solution. During measurements of long-lived reactive protein species (LRPS), the method for determination of H2O2 was modified. Protein solutions (300 μl) in 1 mM PBS were irradiated in 0.65-ml eppendorf polypropylene tubes (SSI, USA). The ratio of the sample volume to the highly diluted “counting solution” was 1:1. All measurements were performed at 25 °C in a dark room. The concentration of hydrogen peroxide used for calibration was quantified by spectrophotometry at 240 nm using a molar absorption coefficient of 43.6 M−1 × cm−1 on a spectrophotometer Cary 100 Scan (Varian, Australia).
Measurement of hydroxyl radicals
The concentrations of OH-radicals were determined using coumarin-3-carboxylic acid (CCA), a fluorescent probe highly specific to •OH (Manevich et al. 1997). The CCA concentration used in the test solutions was 0.5 mM. The intensity of fluorescence was measured on a Cary Eclipse spectrofluorimeter (Varian, Australia) with λex = 400 nm and λem = 450 nm. Calibration for formation of hydroxyl radicals was done using a commercial preparation of 7-OH-CCA. The radiation-chemical yield of •OH was assumed to be equal to 240 nM/Gy (Ward 1988). The measurements were carried out at the Regional Common Use Center “Structural and functional studies of biosystems” of the Institute of Theoretical and Experimental Biophysics, Russian Academy of Sciences.
Immunoenzyme assay for determination of 8-oxoguanine in DNA
The method used for the enzyme immunoassay and the properties of 8-oxoG-specific antibodies have been described in detail previously (Bruskov et al. 1996; Shtarkman et al. 2008). DNA from the salmon sperm dissolved in PBS was used at a concentration of 400 μg/ml. MF was added to the DNA solution just before irradiation. DNA samples were denatured in a boiling water bath for 9 min and cooled on ice. Aliquots of 50 μl were transferred into the wells of immunoassay plates (Costar, USA). DNA was immobilized using a simple dry adsorption procedure with incubation for 3 h at 80 °C until complete evaporation of the solution. To block non-specific sites, 300 μl of a solution containing 1% skimmed milk powder in 0.15 M Tris–HCl buffer, pH 8.5, and 0.15 M NaCl was used. Then the plates were incubated on a plate shaker for 2 h at 37 °C. The formation of an antigen–antibody complex with antibodies (50 μl/well) specific to 8-oxoG (dilution 1:1000) was carried out in a blocking solution by incubation for 2 h at 37 °C. Next, the formation of a complex of the conjugate of antimouse IgG–peroxidase with 8-oxoG-specific antibodies (50 μl/well, 1:1000) was accomplished in a blocking solution by incubation with stirring for 2 h at 37 °C. Samples were washed three times (300 μl/well) with 50 mM Tris–HCl buffer, pH 8.5, and 0.15 M NaCl on a shaker for 3 min. Thereafter, a chromogenic substrate (18.2 mM ABTS) and 10 mM hydrogen peroxide in 75 mM citrate buffer, pH 4.2, were added. The optical density of the samples at 405 nm was measured using a Multiscan FC microplate reader (Thermo Fisher Scientific, Finland). Details of the procedure for the calibration and determination of the concentration of DNA solutions have been published elsewhere (Shtarkman et al. 2008).
Animals and micronucleus test (MN-test)
Males of random-bred white Kv:SHK mice aged 5–6 weeks and weighing 25–29 g (nursery Kryukovo, Russian Academy of Sciences) were used. Animal handling was done according to institutional guidelines for animal care. Mice were kept in a vivarium under controlled conditions of constant room temperature (23 ± 2 °C), ventilation and 12 h day/night cycles, and were given standard commercial mouse feed (Arno, Russia) and drinking water ad libitum.
The cytogenetic damage to the red bone marrow cells of mice during in vivo irradiation was determined from the formation of polychromatophilic erythrocytes (PCE) with micronuclei (MN) (Vral et al. 2011). MF for injection was prepared in physiological saline (150 mM NaCl) at concentrations of 3 and 30 mg/kg bw. MF solutions were injected intraperitoneally (i.p.) to mice 15 min before or after the irradiation with a dose of 1.5 Gy, because linear dose–response relationship between irradiation dose and the number of MN is seen in the dose range of 0–2 Gy (Jagetia and Reddy 2002). Non-irradiated (0 Gy) and irradiated (1.5 Gy) mice, which were injected with the same volume of saline, comprised the control groups. Additional control (non-irradiated mice injected with MF) was absent, because it is already known that at the maximum studied dosage of the drug administered intraperitoneally, MF does not have a genotoxic effect (Amador et al. 2012).
The intraperitoneal route of drug administration was chosen for our study due to its advantages. It is fast and safe, robust, well-tolerated in laboratory animals, ensures rapid and efficient absorption of small molecule pharmacological agents (which include MF (Graham et al. 2011)) from the peritoneal cavity and secures their therapeutic bioavailability. Nowadays, i.p. administration of drugs is generally considered as a justifiable route for exploratory pharmacological studies involving rodents (Al Shoyaib et al. 2020). It is recognized that pharmacokinetics of small molecular weight drugs administered through the i.p. route resembles that of orally administered drugs. Thus, the i.p. route of administration is well-suited for the study of MF as an oral drug and allows one to achieve maximum effect within the framework of a 28-h MN-test.
Mice were sacrificed by cervical dislocation 28 h after irradiation, when irradiated reticulocytes matured. The method for determination of MN was described in detail previously (Schmid 1975) and was used with minor modifications. Briefly, immediately after sacrificing the animal, both femora were removed in toto by cutting through the pelvic bones and below the knee. The bones were freed from muscles by the use of gauze. A micro-tube was filled with serum (40 µl). The needle was inserted a few millimeters deep into the proximal part of the marrow canal. Then the femur was submerged in the serum and by gentle aspirations and flushings the marrow was forced out. Promptly, a small drop (5 µl) of the suspension was put on the end of a slide and spread by pulling the material behind a polished cover glass held at an angle of 45°. The preparations were then air-dried, fixed for 5 min with methanol, air-dried again, and stained for 20 min with 10% Giemsa in phosphate buffer, pH 6.8. The number of MN in PCE was calculated using a light microscope at a magnification of 1000 × . No less than 2000 PCE per mouse were counted, 5 animals per group. All procedures with mice were carried out taking into account the international rules for working with laboratory animals and the requirements of the Commission on Biological Safety and Bioethics of the Institute of Theoretical and Experimental Biophysics of the Russian Academy of Sciences.
Statistical analysis
Data are expressed as mean ± SD. In all in vitro studies, the number of independent experiments (n) is 10, in all in vivo studies it is 5. The background values of hydroxyl radicals, hydrogen peroxide and 8-oxoG are subtracted from the results. Statistical significance was assigned to p < 0.05. The data were subjected to one-way analysis of variance (ANOVA) followed by the Bonferroni post hoc test. Graphical and mathematical processing of the data was performed using the OriginPro 8.1 software package.
Results
Metformin reduces the formation of ROS induced by X-ray-irradiation
Water is the main primary target of IR, and the effect of MF on the yield of water radiolysis products was investigated. MF reduces the formation of hydrogen peroxide upon irradiation of PB and PBS with X-rays. The radiation-chemical yield of H2O2 formation in 0.1 mM phosphate buffer, pH 7.4, with 150 mM NaCl (PBS) is 20% higher than in the 0.1 mM phosphate buffer, pH 7.4 (PB) (Karmanova et al. 2020). MF at a concentration of 250 µM, 1 mM, and 10 mM reduces the formation of hydrogen peroxide in PBS by X-ray-irradiation at a dose of 10 Gy by 25%, 35%, and 55%, respectively (Fig. 1a). With a decrease in the radiation dose, no significant difference in the effect is observed (Fig. 1b).
Effect of MF on concentration and dose dependencies of H2O2 formation in 1 mM phosphate buffer, pH 7.4 (PB), and in 1 mM phosphate buffer, pH 7.4, with 150 mM NaCl (PBS) upon X-ray-irradiation: a Drug concentration dependence, irradiation with 10 Gy; b Dose dependence. * – significantly different from control (p < 0.05, n = 10)
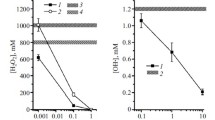
Next, we studied the interaction of MF with •OH, which is the primary product of radiolysis, and interaction of MF directly with H2O2. MF reacts with •OH (Fig. 2a and literature data (Trouillas et al. 2013)), but does not react with H2O2 (Fig. 2b and literature data (Trouillas et al. 2013)). MF at a concentration of 10 mM reduces by 95% the formation of •OH in PB upon X-ray-irradiation at a dose of 5 Gy (Fig. 2a). However, MF (10 mM) added to H2O2 solution (1.5 μM) does not change the concentration of H2O2 significantly within 6 h (Fig. 2b).
The effect of metformin on the formation of long-lived reactive protein species in BSA and BGG solutions under irradiation and on their decay with the formation of ROS
In addition to short-lived ROS, long-lived reactive protein species (LRPS) induced by IR are formed in cells and solutions of various proteins (Dean et al. 1993; Koyama et al. 1998; Ivanov et al. 2019). It has been established that LRPS possess pro-oxidative properties and their decay causes an extension of OS by generation of ROS for a long time (Bruskov et al. 2012).
Albumins are the most abundant serum proteins (Roche et al. 2008), for which the formation of LRPS was previously shown at low BSA concentrations (Bruskov et al. 2012). In this paper, we studied the influence of MF on the process of hydrogen peroxide generation by long-lived reactive species of BSA within 6 h after irradiation at a concentration of BSA that is comparable to physiological one. A BSA solution of 10 mg/ml was used, and this concentration is only 2–3 times lower than the physiological norm for serum albumin.
All the experiments with proteins were carried out in PBS. At a concentration of 10 mg/ml, pro-oxidant properties of BSA at first prevail over its antioxidant properties. Within 1 h after irradiation of BSA solution with 15 Gy, H2O2 was formed by 86% more than in the control (irradiated PBS) (Fig. 3a). However, after 6 h the content of H2O2 decreased to a value of 50% below control. When added to BSA solution before irradiation, MF increased the formation of H2O2 after irradiation only by 15% compared to the control. At the same time, the concentration of H2O2 in a solution of BSA with MF obtained from the calculation of the integral areas for 6 h after irradiation is 35% higher than in the control and is 3% higher than in a solution of BSA without MF (Fig. 3b). This effect does not depend on the concentration of MF in the range from 10 μM to 1 mM. Note that 10 μM is the therapeutic concentration of the drug.
The effect of MF on the formation of long-lived reactive protein species (LRPS) in BSA and BGG under irradiation and on their decay with the formation of ROS: a Formation of H2O2 in a solution of BSA 10 mg/ml in 1 mM PBS upon irradiation with 15 Gy depending on the time after irradiation and the effect of MF (10 μM, 250 μM, and 1 mM) on this process; b Estimation of 6-h area under the “concentration of hydrogen peroxide–time” curve (AUC) for PBS, BSA 10 mg/ml, and BSA 10 mg/ml with MF 250 μM after irradiation with 15 Gy; c Formation of H2O2 in PB upon X-ray-irradiation at a dose of 10 Gy. Impact on this process of sodium sulfate (Na+) or copper sulfate (Cu2+) and the addition of 250 μM MF; d Time dependence of the formation of H2O2 in a solution of BGG 5 mg/ml in PBS after irradiation with 15 Gy and the effect of MF (10 μM, 250 μM, and 1 mM) on this process. Except for b, n = 10 for all the plots
The effect of Cu2+ at concentrations of 5 nM, 5 μM, and 125 μM on the radiation-induced (10 Gy) formation of H2O2 in PB was investigated with the addition of 250 μM MF (Fig. 3c). According to the literature (Tian and Song 2007), MF forms complex with copper at a ratio of 2:1. Sulfate anions only slightly decrease the formation of H2O2. Cu2+ at concentrations of 5 μM and 125 μM reduced the formation of H2O2 by 20% and 60%, respectively. The addition of 250 μM MF significantly enhanced this effect: at 5 μM Cu2+, about 2% of the control was observed, and at 125 μM, background H2O2 values were recorded.
In a solution of BGG (5 mg/ml) irradiated with 15 Gy, H2O2 is formed by 10% less than in the control immediately after irradiation (Fig. 2d). Then, within next 6 h, there is a slow decrease in the content of hydrogen peroxide by another 15% of the originally formed amount (1.35 μM H2O2). MF in the concentration range of 10 μM–1 mM does not affect this process significantly, probably being a less effective scavenger of •OH compared to BGG.
The effect of metformin on radiation-induced formation of 8-oxoguanine in a DNA solution
The most sensitive biomarker of oxidative damage to DNA is the formation of 8-oxoG (Kasai 1997). High-molecular DNA from salmon sperm was used to determine the genoprotective properties of MF in vitro upon irradiation of a DNA solution with 10 Gy of X-rays. MF in the range from 0.05–0.25 mM reduces the formation of 8-oxoG in direct proportion to the concentration of MF, reaching a maximum effect (36% of control) at 0.25 mM (Fig. 4). But with an increase in the concentration of MF to 0.5–1 mM, this effect weakens to the level of 75% of control and its saturation occurs.
Radiomitigatory properties of metformin
Hematopoietic system is one of the most sensitive to the effects of IR, and the radiosensitivity of red bone marrow cells is extremely high. The micronucleus test allows one to analyze in vivo the damage to nuclear DNA after irradiation of animals. The MN-test is one of the common methods for studying the genotoxicity of IR (Bagheri et al. 2018). We studied the effect of MF administered intraperitoneally to mice before or after irradiation with 1.5 Gy on the frequency of formation of PCE with MN in red bone marrow of animals (Fig. 5). MF (3 and 30 mg/kg bw) exhibits genoprotective effect, reducing the percentage of PCE with MN by 65% and 75%, respectively, when administered to mice i.p. 15 min after irradiation at a dose of 1.5 Gy (Fig. 5a). At the same time, introduction of MF (30 mg/kg bw) 15 min before irradiation does not have statistically significant effect (Fig. 5b). Thus, MF is effective when administered immediately post-irradiation, consistent with survival data gained in mice (Abdullaev et al. 2018).
Influence of MF on the frequency of polychromatophilic erythrocytes (PCE) with micronuclei (MN) in bone marrow of X-ray-irradiated mice: a Dose dependence of the effect of MF (3 or 30 mg/kg bw, administered i.p. after irradiation); b Dependence of the effect of MF (30 mg/kg bw, i.p.) on the time of its administration to mice relative to irradiation (before or after irradiation with 1.5 Gy). * – significantly different from intact and irradiated controls (p < 0.05, n = 5)
Discussion
Aqueous solutions are the main primary target of the damaging effects of IR on living systems (Halliwell and Gutteridge 2015; Bruskov et al. 2020). During the radiolysis of water, the following reaction products are formed – hydroxyl radicals, hydrogen atoms (radicals), protons, hydrated electrons, hydrogen molecules and hydrogen peroxide (1):
Three ways of formation of H2O2 are possible: recombination of hydroxyl radicals (2), reaction of a hydrogen atom with a hydroperoxide radical (4), and dismutation of hydroperoxide radicals (3):
Unlike other short-lived radicals, H2O2 is a long-lived form of ROS. Hydrogen peroxide in cells and tissues of the body performs a signaling-regulatory role associated with the maintaining of redox homeostasis.
Influence of MF on the radiation-induced generation of hydrogen peroxide, starting at about 250 μM, is probably due to the formation of less •OH, recombination of which leads to the formation of hydrogen peroxide (reaction 2).
Redox-active metal ions, including Cu2+ (Argirova and Ortwerth 2003), can catalyze Haber–Weiss reaction leading to the formation of •OH from H2O2 and superoxide (5):
The complex of MF with Cu2+ enhances the reaction of H2O2 decomposition and the production of •OH. MF forms with Cu2+ ions delocalized electronic planar ring structures (Tian and Song 2007; Vasantha et al. 2018). Such a structure can substantially strengthen the catalytic properties of Cu2+ ions as judged from our results. There is some evidence for the ability of the MF–copper complex to form a catalytic cycle: unknown Cu2+-containing species acts as a reducing agent to reduce the N-hydroxy imino group, the oxidation product of the imino group, to its initial form. The authors suggest that it may be Cu2+-oxo-coordination complexes with ROS (Halime et al. 2010), such as superoxide anion and hydroxyl radical (Tian and Song 2007).
Besides the aquatic medium, the next main targets of radiation exposure are proteins containing variety of reactive amino acid residues. Proteins, being effective antioxidants and scavengers of reactive products, protect from oxidative damage other biological structures, including nucleic acids, lipids, and others. Albumins and globulins play an important role in the metabolic processes in the body due to their high content. These proteins are likely implicated in some signaling pathways and may influence the generation of ROS by forming LRPS, which gradually decay with the formation of ROS for extended time after irradiation, thus prolonging OS (Simpson et al. 1992; Bruskov et al. 2012; Ivanov et al. 2017, 2019). Radiation-induced damage triggers changes in cellular redox homeostasis, receptor signaling (Davies 2016) and activates the expression of genes related to antioxidant protection and repair processes (Petrou and Terzidaki 2017). ROS generated by LRPS can participate in OS signaling and in regulatory processes (Zhang et al. 2016a). Formation of LRPS through the long-lived peroxyl protein radicals is described previously (Ivanov et al. 2019).
The transport of small molecules and drugs as well as chelation of metal ions are among the main biological functions of serum albumin (Argirova and Ortwerth 2003; Roche et al. 2008). Protein-bound metal ions can be a source of oxidative damage to biomacromolecules. In a solution, BSA exhibits both pro-oxidant and antioxidant properties after irradiation. Albumin contains six methionine residues, Cys34, and the redox-active disulfide Cys392/Cys438 (Roche et al. 2008; Goncharov et al. 2015), which are capable of scavenging radicals. The N-terminal region of human albumin AspAlaHisLys in complex with copper ions has a pronounced superoxide dismutase activity (Kato et al. 2014) facilitated by oxidation of the SH group of Cys34 (Gryzunov et al. 2003; Belinskaia et al. 2020). It is this site that is credited with both antioxidant (Gryzunov et al. 2003) and pro-oxidant (Gryzunov et al. 2003; Goncharov et al. 2015; Belinskaia et al. 2020) activities, which is also confirmed by our results. Pro-oxidant activity of BSA, which is observed in the first hour after irradiation, can be caused by its Cu-binding site along with other metal-binding sites (Roche et al. 2008) and by the mechanism of LRPS pro-oxidant action described above, which is common to all proteins. The proposed mechanism for the production of ROS by LRPS occurs through superoxide/hydroperoxide radicals, so that MF cannot directly affect the formation of H2O2 by LRPS, but it has a clear modifying effect on the process.
MF is able to chelate the metal ions contained in BSA (Argirova and Ortwerth 2003; Ali et al. 2020). In our experiments, MF on addition to BSA solution before irradiation even at a concentration as low as 10 μM decreased the content of H2O2 within 1 h after irradiation and further blocked the catalytic decomposition of H2O2 up to 6 h. The observed effect of MF can be partly explained by the following mechanism. MF binds and displaces Cu2+ ions from BSA, after which the resulting complex MF–Cu2+ begins to actively decompose H2O2 formed as a result of radiolysis and produced by LRPS. The resulting hydroxyl radicals react with MF and the complex disintegrates. This process can occur in the vicinity of the BSA active center, which will lead to its oxidative damage and loss of function.
Our study shows that BGG is capable of both rapid ROS interception during irradiation and further slow elimination of H2O2, similarly to Cu2+-binding BSA. The addition of MF to the solution does not modify the antioxidant effect of BGG, probably due to the absence of direct interaction between MF and gamma-globulins. Again, the ability of MF to intercept •OH is extremely low compared to BGG. Available literature lacks data on the molecular interrelations between MF and gamma-globulins (immunoglobulins, Ig). There are only data on the signaling effect of MF on Ig levels: induction of intestinal IgA (Ustinova et al. 2019), decrease of serum IgG in inflammation (Kim et al. 2019), arthritis (Son et al. 2014), and in autoimmune diseases (Singh et al. 2020).
The gene-protective effect of MF that we observed suggests a direct interaction of MF with DNA. MF itself does not directly cause DNA oxidation (Machini et al. 2019). The positive charges of MF can bind to the negative charges of DNA phosphate groups (Mondal et al. 2018). MF interacts with the DNA helix mainly by the groove binding (Shahabadi and Heidari 2012; Mondal et al. 2018). MF binds to double-stranded (ds) DNA with the formation of a condensed, more compact structure, in which purine bases are less prone for oxidation. These data were obtained at a concentration ratio (25 μM MF and 50 μg/ml dsDNA), which is comparable to the concentration ratio corresponding to the maximum gene-protective effect of MF (250 μM MF and 400 μg/ml dsDNA) observed by us in our study. Later, after a week of incubation, the authors found that the structure of MF–dsDNA complex changed, which caused local unwinding of dsDNA. Presumably, this process also took place in our case. Most likely, MF manifests its protective function both by scavenging •OH and by stabilizing the DNA structure. With an excess of MF, its stabilizing effect is leveled, and •OH interception is not sufficient to protect DNA from the oxidative damage.
Primarily, the antioxidant and gene-protective properties of MF are important for mitochondrial DNA (mtDNA). The damage to mtDNA and mutations in mitochondrial genes encoding the subunits of respiratory enzymes reduce the production of ATP, which is necessary for repair processes, and entail further injuries to the mitochondria (Richter 1992). MF is considered precisely as a mitochondria-targeted radiomitigator (Abdullaev et al. 2018), which inhibits the respiratory chain and prevents hyper-production of ROS. Our results indicate that MF has the potential to directly protect mtDNA from oxidative damage.
MF has been shown to be able to protect cells from IR-induced damage both in vitro in cell cultures (Cheki et al. 2016) and in vivo (Xu et al. 2015; Abdullaev et al. 2018). In MN-test, we used low doses of MF (3–30 mg/kg bw, i.p.), which are 7–10 times lower than those used orally (Xu et al. 2015; Abdullaev et al. 2018) and 3 times lower than the dose that was demonstrated to lack in vivo genotoxicity and does not cause MN formation in mouse bone marrow cells (Amador et al. 2012). The result we obtained at a dose of 30 mg/kg bw is comparable with the previous results (Abdullaev et al. 2018). MN-test has shown that apart from its direct antioxidant activity, MF exhibits pronounced radiomitigatory properties when administered post-irradiation. Conceivably, the signaling is the most important component of the radiomitigatory effect of MF, which is also indicated in the literature (Miller et al. 2014; Xu et al. 2015).
Research into the effect of MF directly on erythropoiesis was not the aim of our study; hence, the NCE/PCE ratio was not calculated. Though the properties of MF have been extensively investigated, the published literature contains only few works devoted to the impact of MF on erythropoiesis. MF ameliorates defective erythropoiesis in models of Diamond-Blackfan anemia (Wilkes et al. 2020) and Fanconi anemia (Zhang et al. 2016b). MF increased the size of the hematopoietic stem cell compartment and enhanced quiescence in hematopoietic stem and progenitor cells. However, MF improved peripheral blood counts only after 6 months of therapy (Zhang et al. 2016b).
Conclusion
Our study has shown that MF alleviates radiation exposure induced oxidative stress and exerts genoprotective and radiomitigatory properties when administered to animals after irradiation. MF may prove to be a useful treatment option to allay the adverse side effects of radiotherapy in cancer patients. Besides, MF is attractive for use in the medical management of radiation accidents. The efficiency of MF as a protective agent against the harmful effects of radiation exposure depends on many parameters: dose of IR, dosage of MF, dosing schedule, method of drug administration (Abdullaev et al. 2018), and others. Therefore, further large-scale studies, both experimental and clinical, are warranted.
Data availability
All the data supporting the findings of this study are included in this published article.
References
Abdullaev S, Minkabirova G, Karmanova E, Bruskov V, Gaziev A (2018) Metformin prolongs survival rate in mice and causes increased excretion of cell-free DNA in the urine of X-irradiated rats. Mutat Res 831:13–18. https://doi.org/10.1016/j.mrgentox.2018.05.006
Al Shoyaib A, Archie SR, Karamyan VT (2020) Intraperitoneal route of drug administration: should it be used in experimental animal studies? Pharm Res 37:12. https://doi.org/10.1007/s11095-019-2745-x
Ali R, Alminderej FM, Saleh SM (2020) A simple, quantitative method for spectroscopic detection of metformin using gold nanoclusters. Spectrochim Acta A Mol Biomol Spectrosc 241:118744. https://doi.org/10.1016/j.saa.2020.118744
Amador RR, Longo JPF, Lacava ZG, Dorea JG, Almeida Santos M de F (2012) Metformin (dimethyl-biguanide) induced DNA damage in mammalian cells. Genet Mol Biol 35:153–158. https://doi.org/10.1590/s1415-47572011005000060
Argirova MD, Ortwerth BJ (2003) Activation of protein-bound copper ions during early glycation: study on two proteins. Arch Biochem Biophys 420:176–184. https://doi.org/10.1016/j.abb.2003.09.005
Bagheri H, Rezapour S, Najafi M, Motevaseli E, Shekarchi B, Cheki M et al (2018) Protection against radiation-induced micronuclei in rat bone marrow erythrocytes by curcumin and selenium L-methionine. Iran J Med Sci 43:645–652
Belinskaia DA, Voronina PA, Shmurak VI, Vovk MA, Batalova AA, Jenkins RO et al (2020) The universal soldier: enzymatic and non-enzymatic antioxidant functions of serum albumin. Antioxidants 9:966. https://doi.org/10.3390/antiox9100966
Bramante CT, Ingraham NE, Murray TA, Marmor S, Hovertsen S, Gronski J et al (2021) Metformin and risk of mortality in patients hospitalized with COVID-19: a retrospective cohort analysis. Lancet Healthy Longev 2:e34–e41. https://doi.org/10.1016/s2666-7568(20)30033-7
Bruskov VI, Gaziev AI, Malakhova LV, Mantsygin YA, Morenkov OS (1996) Monoclonal antibodies to 8-oxo-2’-deoxyguanosine (8-hydroxyguanosine): characterization and use in determining damage to DNA by reactive oxygen species. Biochem Mosc 61:535–540
Bruskov VI, Karp OE, Garmash SA, Shtarkman IN, Chernikov AV, Gudkov SV (2012) Prolongation of oxidative stress by long-lived reactive protein species induced by X-ray radiation and their genotoxic action. Free Radic Res 46:1280–1290. https://doi.org/10.3109/10715762.2012.709316
Bruskov VI, Chernikov AV, Ivanov VE, Karmanova EE, Gudkov SV (2020) Formation of the reactive species of oxygen, nitrogen, and carbon dioxide in aqueous solutions under physical impacts. Phys Wave Phenom 28:103–106. https://doi.org/10.3103/S1541308X2002003X
Campbell JM, Bellman SM, Stephenson MD, Lisy K (2017) Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: a systematic review and meta-analysis. Ageing Res Rev 40:31–44. https://doi.org/10.1016/j.arr.2017.08.003
Cheki M, Shirazi A, Mahmoudzadeh A, Bazzaz JT, Hosseinimehr SJ (2016) The radioprotective effect of metformin against cytotoxicity and genotoxicity induced by ionizing radiation in cultured human blood lymphocytes. Mutat Res 809:24–32. https://doi.org/10.1016/j.mrgentox.2016.09.001
Chen G, Xu S, Renko K, Derwahl M (2012) Metformin inhibits growth of thyroid carcinoma cells, suppresses self-renewal of derived cancer stem cells, and potentiates the effect of chemotherapeutic agents. J Clin Endocrinol Metab 97:E510–E520. https://doi.org/10.1210/jc.2011-1754
Cheng T, Wang C, Lu Q, Cao Y, Yu W, Li W et al (2022) Metformin inhibits the tumor-promoting effect of low-dose resveratrol, and enhances the anti-tumor activity of high-dose resveratrol by increasing its reducibility in triple negative breast cancer. Free Radic Biol Med 180:108–120. https://doi.org/10.1016/j.freeradbiomed.2022.01.010
Davies MJ (2016) Protein oxidation and peroxidation. Biochem J 473:805–825. https://doi.org/10.1042/BJ20151227
Dean RT, Gieseg S, Davies MJ (1993) Reactive species and their accumulation on radical-damaged proteins. Trends Biochem Sci 18:437–441. https://doi.org/10.1016/0968-0004(93)90145-d
DeFronzo RA, Goodman AM (1995) Efficacy of metformin in patients with non-insulin-dependant diabetes mellitus. N Engl J Med 333:541–549. https://doi.org/10.1056/nejm199508313330902
Goncharov NV, Belinskaya DA, Razygraev AV, Ukolov AI (2015) On the enzymatic activity of albumin. Russ J Bioorg Chem 41:131–144. https://doi.org/10.1134/s1068162015020041
Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK et al (2011) Clinical pharmacokinetics of metformin. Clin Pharmacokinet 50:81–98. https://doi.org/10.2165/11534750-000000000-00000
Gryzunov YA, Arroyo A, Vigne JL, Zhao Q, Tyurin VA, Hubel CA et al (2003) Binding of fatty acids facilitates oxidation of cysteine-34 and converts copper-albumin complexes from antioxidants to prooxidants. Arch Biochem Biophys 413:53–66. https://doi.org/10.1016/s0003-9861(03)00091-2
Halime Z, Kieber-Emmons MT, Qayyum MF, Mondal B, Gandhi T, Puiu SC et al (2010) Heme–copper–dioxygen complexes: toward understanding ligand-environmental effects on the coordination geometry, electronic structure, and reactivity. Inorg Chem 49:3629–3645. https://doi.org/10.1021/ic9020993
Halliwell B, Gutteridge JM (2015) Free radicals in biology and medicine. Oxford University Press, London
Han Y, Xie H, Liu Y, Gao P, Yang X, Shen Z (2019) Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: a systematic review and an updated meta-analysis. Cardiovasc Diabetol 18:1–16. https://doi.org/10.1186/s12933-019-0900-7
Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S et al (2010) Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab 11:554–565. https://doi.org/10.1016/j.cmet.2010.04.001
Honjo S, Ajani JA, Scott AW et al (2014) Metformin sensitizes chemotherapy by targeting cancer stem cells and the mTOR pathway in esophageal cancer. Int J Oncol 45:567–574. https://doi.org/10.3892/ijo.2014.2450
Ivanov VE, Usacheva AM, Chernikov AV, Bruskov VI, Gudkov SV (2017) Formation of long-lived reactive species of blood serum proteins induced by low-intensity irradiation of helium-neon laser and their involvement in the generation of reactive oxygen species. J Photochem Photobiol b: Biology 176:36–43. https://doi.org/10.1016/j.jphotobiol.2017.09.012
Ivanov VE, Karp OE, Bruskov VI, Andreev SN, Bunkin NF, Gudkov SV (2019) Formation of long-lived reactive products in blood serum under heat treatment and low-intensity laser irradiation, their role in hydrogen peroxide generation and DNA damage. Ind J Biochem Biophys 56:214–223. http://op.niscair.res.in/index.php/IJBB/article/view/27641
Jagetia GC, Reddy TK (2002) The grapefruit flavanone naringin protects against the radiation-induced genomic instability in the mice bone marrow: a micronucleus study. Mutat Res 519:37–48. https://doi.org/10.1016/s1383-5718(02)00111-0
Kalender A, Selvaraj A, Kim SY, Gulati P, Brule S, Viollet B et al (2010) Metformin, independent of AMPK, inhibits mTORC1 in a Rag GTPase-dependent manner. Cell Metab 11:390–401. https://doi.org/10.1016/j.cmet.2010.03.014
Karmanova EE, Chernikov AV, Usacheva AM, Bruskov VI (2020) Antioxidant and gene-protective properties of ethylmethylhydroxypyridine succinate (Mexidol) in X-ray irradiation. Pharm Chem J 54:673–677. https://doi.org/10.1007/s11094-020-02255-2
Kasai H (1997) Analysis of a form of oxidative DNA damage, 8-hydroxy-2’-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat Res 387:147–163. https://doi.org/10.1016/s1383-5742(97)00035-5
Kato R, Akiyama M, Kawakami H, Komatsu T (2014) Superoxide dismutase activity of the naturally occurring human serum albumin-copper complex without hydroxyl radical formation. Chem Asian J 9:83–86. https://doi.org/10.1002/asia.201301285
Kim JW, Kim SM, Park JS, Hwang SH, Choi J, Jung KA et al (2019) Metformin improves salivary gland inflammation and hypofunction in murine Sjogren’s syndrome. Arthritis Res Ther 21:136. https://doi.org/10.1186/s13075-019-1904-0
Koritzinsky M (2015) Metformin: a novel biological modifier of tumor response to radiation therapy. Int J Radiat Oncol Biol Phys 93:454–464. https://doi.org/10.1016/j.ijrobp.2015.06.003
Koyama S, Kodama S, Suzuki K, Matsumoto T, Miyazaki T, Watanabe M (1998) Radiation-induced long-lived radicals which cause mutation and transformation. Mutat Res 421:45–54. https://doi.org/10.1016/s0027-5107(98)00153-5
Machini WBS, Fernandes IPG, Oliveira-Brett AM (2019) Antidiabetic drug metformin oxidation and in situ interaction with dsDNA using a dsDNA-electrochemical biosensor. Electroanalysis 31:1977–1987. https://doi.org/10.1002/elan.201900162
Manevich Y, Held KD, Biaglow JE (1997) Coumarin-3-carboxylic acid as a detector for •OH generated chemically and by gamma radiation. Radiat Res 148:580–591. https://doi.org/10.2307/3579734
Martin-Castillo B, Dorca J, Vazquez-Martin A, Oliveras-Ferraros C, Lopez-Bonet E, Garcia M et al (2010) Incorporating the antidiabetic drug metformin in HER2-positive breast cancer treated with neoadjuvant chemotherapy and trastuzumab: an ongoing clinical translational research experience at the Catalan Institute of Oncology. Ann Oncol 21:187–189. https://doi.org/10.1093/annonc/mdp494
Miller RC, Murley JS, Grdina DJ (2014) Metformin exhibits radiation countermeasures efficacy when used alone or in combination with sulfhydryl containing drugs. Radiat Res 181:464–470. https://doi.org/10.1667/rr13672.1
Mondal S, Samajdar RN, Mukherjee S, Bhattacharyya AJ, Bagchi B (2018) Unique features of metformin: a combined experimental, theoretical, and simulation study of its structure, dynamics, and interaction energetics with DNA grooves. J Phys Chem B 122:2227–2242. https://doi.org/10.1021/acs.jpcb.7b11928
Mortezaee K, Shabeeb D, Musa AE, Najafi M, Farhood B (2019) Metformin as a radiation modifier; implications to normal tissue protection and tumor sensitization. Cur Clin Pharm 14:41–53. https://doi.org/10.2174/1574884713666181025141559
Najafi M, Cheki M, Rezapoor S, Geraily G, Motevaseli E, Carnovale C et al (2018) Metformin: prevention of genomic instability and cancer: a review. Mutat Res 827:1–8. https://doi.org/10.1016/j.mrgentox.2018.01.007
Obrador E, Salvador R, Villaescusa JI, Soriano JM, Estrela JM, Montoro A (2020) Radioprotection and radiomitigation: from the bench to clinical practice. Biomedicines 8(11):461. https://doi.org/10.3390/biomedicines8110461
Owen MR, Doran E, Halestrap AP (2000) Evidence that metformin exerts its antidiabetic effects through inhibition of complex I of the mitochondrial respiratory chain. Biochem J 348:607–614. https://doi.org/10.1042/bj3480607
Pernicova I, Korbonits M (2014) Metformin – mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol 10:143–156. https://doi.org/10.1038/nrendo.2013.256
Petrou AL, Terzidaki AA (2017) A meta-analysis and review examining a possible role for oxidative stress and singlet oxygen in diverse diseases. Biochem J 474:2713–2731. https://doi.org/10.1042/bcj20161058
Pietrocola F, Kroemer G (2017) Metformin: a metabolic modulator. Oncotarget 8:9017–9020. https://doi.org/10.18632/oncotarget.14794
Piskovatska V, Storey KB, Vaiserman AM, Lushchak O (2020) The use of metformin to increase the human healthspan. In: Guest P (ed) Reviews on new drug targets in age-related disorders. Advances in experimental medicine and biology, vol 1260. Springer, Cham, pp 319–332. https://doi.org/10.1007/978-3-030-42667-5_13
Richter C (1992) Reactive oxygen and DNA damage in mitochondria. Mutat Res 275:249–255. https://doi.org/10.1016/0921-8734(92)90029-o
Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E (2008) The antioxidant properties of serum albumin. FEBS Lett 582:1783–1787. https://doi.org/10.1016/j.febslet.2008.04.057
Schernthaner G, Brand K, Bailey CJ (2022) Metformin and the heart: update on mechanisms of cardiovascular protection with special reference to comorbid type 2 diabetes and heart failure. Metabolism 130:155160. https://doi.org/10.1016/j.metabol.2022.155160
Schmid W (1975) The micronucleus test. Mutat Res 31:9–15. https://doi.org/10.1016/0165-1161(75)90058-8
Shahabadi N, Heidari L (2012) Binding studies of the antidiabetic drug metformin to calf thymus DNA using multispectroscopic methods. Spectrochim Acta A Mol Biomol Spectrosc 97:406–410. https://doi.org/10.1016/j.saa.2012.06.044
Shtarkman IN, Gudkov SV, Chernikov AV, Bruskov VI (2008) Effect of amino acids on X-ray-induced hydrogen peroxide and hydroxyl radical formation in water and 8-oxoguanine in DNA. Biochem Mosc 73:470–478. https://doi.org/10.1134/s0006297908040135
Simpson JA, Narita S, Gieseg S, Gebicki S, Gebicki JM, Dean RT (1992) Long-lived reactive species on free-radical-damaged proteins. Biochem J 282:621–624. https://doi.org/10.1042/bj2820621
Singh SS, Naber A, Dotz V, Schoep E, Memarian E, Slieker RC et al (2020) Metformin and statin use associate with plasma protein N-glycosylation in people with type 2 diabetes. BMJ Open Diabetes Res Care 8:e001230. https://doi.org/10.1136/bmjdrc-2020-001230
Son HJ, Lee J, Lee SY, Kim EK, Park MJ, Kim KW et al (2014) Metformin attenuates experimental autoimmune arthritis through reciprocal regulation of Th17/Treg balance and osteoclastogenesis. Mediat Inflamm 2014:973986. https://doi.org/10.1155/2014/973986
Tajabadi E, Javadi A, Azar NA, Najafi M, Shirazi A, Shabeeb D et al (2020) Radioprotective effect of a combination of melatonin and metformin on mice spermatogenesis: a histological study. Int J Reprod Biomed 18:1073. https://doi.org/10.18502/ijrm.v18i12.8029
Tian XJ, Song JF (2007) Catalytic action of copper (II) ion on electrochemical oxidation of metformin and voltammetric determination of metformin in pharmaceuticals. J Pharm Biomed Anal 44:1192–1196. https://doi.org/10.1016/j.jpba.2007.04.014
Toyama EQ, Herzig S, Courchet J, Lewis TL, Loson OC, Hellberg K et al (2016) AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 351:275–281. https://doi.org/10.1126/science.aab4138
Triggle CR, Mohammed I, Bshesh K, Marei I, Ye K, Ding H et al (2022) Metformin: is it a drug for all reasons and diseases? Metabolism 133:155223. https://doi.org/10.1016/j.metabol.2022.155223
Trouillas P, Marchetti C, Bonnefont-Rousselot D, Lazzaroni R, Jore D, Gardes-Albert M et al (2013) Mechanism of one-electron oxidation of metformin in aqueous solution. Phys Chem Chem Phys 15:9871–9878. https://doi.org/10.1039/c3cp50602a
Ustinova M, Silamikelis I, Kalnina I, Ansone L, Rovite V, Elbere I (2019) Metformin strongly affects transcriptome of peripheral blood cells in healthy individuals. PLoS One 14:e0224835. https://doi.org/10.1371/journal.pone.0224835
Vasantha P, Shekhar B, Anantha Lakshmi PV (2018) Copper-metformin ternary complexes: thermal, photochemosensitivity and molecular docking studies. Mater Sci Eng C Mater Biol Appl 90:621–633. https://doi.org/10.1016/j.msec.2018.04.052
Vral A, Fenech M, Thierens H (2011) The micronucleus assay as a biological dosimeter of in vivo ionising radiation exposure. Mutagenesis 26:11–17. https://doi.org/10.1093/mutage/geq078
Wang B, Dong J, Xiao H, Li Y, Jin Y, Ming Cui M et al (2020) Metformin fights against radiation-induced early developmental toxicity. Sci Total Environ 732:139274. https://doi.org/10.1016/j.scitotenv.2020.139274
Ward JF (1988) DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation and reparability. Prog Nucleic Acid Res Mol Biol 35:95–125. https://doi.org/10.1016/s0079-6603(08)60611-x
Wilkes MC, Siva K, Varetti G, Mercado J, Wentworth EP, Perez CA et al (2020) Metformin-induced suppression of Nemo-like kinase improves erythropoiesis in preclinical models of Diamond-Blackfan anemia through induction of miR-26a. Exp Hematol 91:65–77. https://doi.org/10.1016/j.exphem.2020.09.187
Xu G, Wu H, Zhang J, Li D, Wang Y, Wang Y et al (2015) Metformin ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic Biol Med 87:15–25. https://doi.org/10.1016/j.freeradbiomed.2015.05.045
Yahyapour R, Amini P, Saffar H, Motevaseli E, Farhood B, Pooladvand V et al (2019) Protective effect of metformin, resveratrol and alpha-lipoic acid on radiation-induced pneumonitis and fibrosis: a histopathological study. Curr Drug Res Rev 11:111–117. https://doi.org/10.2174/2589977511666191018180758
Yi Y, Zhang W, Yi J, Xiao Z-X (2019) Role of p53 family proteins in metformin anti-cancer activities. J Cancer 10:2434–2442. https://doi.org/10.7150/jca.30659
Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y et al (2016a) ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev 2016:4350965. https://doi.org/10.1155/2016/4350965
Zhang QS, Tang W, Deater M, Phan N, Marcogliese AN, Li H et al (2016b) Metformin improves defective hematopoiesis and delays tumor formation in Fanconi anemia mice. Blood 128:2774–2784. https://doi.org/10.1182/blood-2015-11-683490
Funding
This study was supported by the Ministry of Science and Higher Education of the Russian Federation (contract No. 075-01027-22-00 with the Institute of Theoretical and Experimental Biophysics, Russian Academy of Sciences) and by the Russian Science Foundation (RSF) grant No. 22-63-00082 (“Interdisciplinary Projects”).
Author information
Authors and Affiliations
Contributions
VB: conceptualization, supervision and project administration; VB and NP: methodology; MS: validation; EK and VI: investigation; EK and MS: data curation; EK, NP, and VI: formal analysis; EK: writing original draft, visualization; AC: interpretation of the data and final editing; all the authors: review and editing. All the authors have read and agreed to the final version of the manuscript. The authors declare that all the data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Commission on Biological Safety and Bioethics of the Institute of Theoretical and Experimental Biophysics of the Russian Academy of Sciences (protocol No. 25/2021 of February 09, 2021).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karmanova, E.E., Chernikov, A.V., Popova, N.R. et al. Metformin mitigates radiation toxicity exerting antioxidant and genoprotective properties. Naunyn-Schmiedeberg's Arch Pharmacol 396, 2449–2460 (2023). https://doi.org/10.1007/s00210-023-02466-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02466-w