Abstract
Diabetic nephropathy (DN) is a serious complication of diabetes mellitus (DM), which is a major public health problem in the world. To reveal the metabolic changes associated with DN, we analyzed the serum, urine, and renal extracts obtained from control and streptozotocin (STZ)-induced DN rats by 1H NMR-based metabonomics and multivariate data analysis. A significant difference between control and DN rats was revealed in metabolic profiles, and we identified several important DN-related metabolites including increased levels of allantoin and uric acid (UA) in the DN rats, suggesting that disturbed purine metabolism may be involved in the DN. Combined with conventional histological and biological methods, we further demonstrated that xanthine oxidase (XO), a key enzyme for purine catabolism, was abnormally activated in the kidney of diabetic rats by hyperglycemia. The highly activated XO increased the level of intracellular ROS, which caused renal injury by direct oxidative damage to renal cells, and indirect inducing inflammatory responses via activating NF-κB signaling pathway. Our study highlighted that metabonomics is a promising tool to reveal the metabolic changes and the underlying mechanism involved in the pathogenesis of DN.

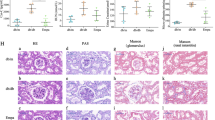
In this study, we performed 1H NMR-based metabonomic to analyze the serum, urine, and renal extracts in a rat model of diabetic nephropathy (DN), and identified the disturbed purine metabolism and its underlying xanthine oxidase induced oxidative stress and inflammation was involved in the pathogenesis of DN








Similar content being viewed by others
References
Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T (2005) Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 28:164–176
Stanton RC (2014) Clinical challenges in diagnosis and management of diabetic kidney disease. Am J Kidney Dis 63:S3–S21
Ritz E, Zeng XX, Rychlik I (2011) Clinical manifestation and natural history of diabetic nephropathy. Contrib Nephrol 170:19–27
Kanwar YS, Sun L, Xie P, Liu FY, Chen S (2011) A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol 6:395–423
Dronavalli S, Duka I, Bakris GL (2008) The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab 4:444–452
Singh DK, Winocour P, Farrington K (2011) Oxidative stress in early diabetic nephropathy: fueling the fire. Nat Rev Endocrinol 7:176–184
Nicholson JK, Lindon JC (2008) Systems biology: metabonomics. Nature 455:1054–1056
Rezzi S, Ramadan Z, Fay LB, Kochhar S (2007) Nutritional metabonomics: applications and perspectives. J Proteome Res 6:513–525
Dieterle F, Riefke B, Schlotterbeck G, Ross A, Senn H, Amberg (2011) A NMR and MS methods for metabonomics. Methods Mol Biol 691:385–415
Makinen VP, Soininen P, Forsblom C, Parkkonen M, Ingman P, Kaski K, Groop PH, Ala-Korpela M (2008) 1H NMR metabonomics approach to the disease continuum of diabetic complications and premature death. Mol Syst Biol 4:167
Hirayama A, Nakashima E, Sugimoto M, Akiyama S, Sato W, Maruyama S, Matsuo S, Tomita M, Yuzawa Y, Soga T (2012) Metabolic profiling reveals new serum biomarkers for differentiating diabetic nephropathy. Anal Bioanal Chem 404:3101–3109
Pena MJ, Lambers Heerspink HJ, Hellemons ME, Friedrich T, Dallmann G, Lajer M, Bakker SJ, Gansevoort RT, Rossing P, de Zeeuw D, Roscioni SS (2014) Urine and plasma metabolites predict the development of diabetic nephropathy in individuals with Type 2 diabetes mellitus. Diabet Med 31:1138–1147
Zhao L, Gao H, Lian F, Liu X, Zhao Y, Lin D (2011) 1H-NMR-based metabonomic analysis of metabolic profiling in diabetic nephropathy rats induced by streptozotocin. Am J Physiol Ren Physiol 300:F947–F956
Beckonert O, Keun HC, Ebbels TM, Bundy J, Holmes E, Lindon JC, Nicholson JK (2007) Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat Protoc 2:2692–2703
Weljie AM, Newton J, Mercier P, Carlson E, Slupsky CM (2006) Targeted profiling: quantitative analysis of 1H NMR metabolomics data. Anal Chem 78:4430–4442
Wang D, Liu J, He S, Wang C, Chen Y, Yang L, Liu F, Ren Y, Tian H, Yang G, Liao G, Li L, Shi M, Yuan Y, Zhao J, Cheng J, Lu Y (2014) Assessment of early renal damage in diabetic rhesus monkeys. Endocrine 47:783–792
Bain JR, Stevens RD, Wenner BR, Ilkayeva O, Muoio DM, Newgard CB (2009) Metabolomics applied to diabetes research: moving from information to knowledge. Diabetes 58:2429–2443
Liu J, Wang D, Chen Y, Sun H, He S, Wang C, Yang G, Shi M, Zhang J, Ren Y, Wang L, Lu Y, Cheng J (2013) 1H NMR-based metabonomic analysis of serum and urine in a nonhuman primate model of diabetic nephropathy. Mol Biosyst 9:2645–2652
Zoppini G, Targher G, Chonchol M, Ortalda V, Abaterusso C, Pichiri I, Negri C, Bonora E (2012) Serum uric acid levels and incident chronic kidney disease in patients with type 2 diabetes and preserved kidney function. Diabetes Care 35:99–104
Hovind P, Rossing P, Tarnow L, Johnson RJ, Parving HH (2009) Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: an inception cohort study. Diabetes 58:1668–1671
George J, Struthers AD (2009) Role of urate, xanthine oxidase and the effects of allopurinol in vascular oxidative stress. Vasc Health Risk Manag 5:265–272
Doehner W, Landmesser U (2011) Xanthine oxidase and uric acid in cardiovascular disease: clinical impact and therapeutic options. Semin Nephrol 31:433–440
Marklund N, Ostman B, Nalmo L, Persson L, Hillered L (2000) Hypoxanthine, uric acid and allantoin as indicators of in vivo free radical reactions. Description of a HPLC method and human brain microdialysis data. Acta Neurochir (Wien) 142:1135–1141
Bravard A, Bonnard C, Durand A, Chauvin MA, Favier R, Vidal H, Rieusset J (2011) Inhibition of xanthine oxidase reduces hyperglycemia-induced oxidative stress and improves mitochondrial alterations in skeletal muscle of diabetic mice. Am J Physiol Endocrinol Metab 300:E581–E591
Desco MC, Asensi M, Marquez R, Martinez-Valls J, Vento M, Pallardo FV, Sastre J, Vina J (2002) Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol. Diabetes 51:1118–1124
Rajesh M, Mukhopadhyay P, Batkai S, Mukhopadhyay B, Patel V, Hasko G, Szabo C, Mabley JG, Liaudet L, Pacher P (2009) Xanthine oxidase inhibitor allopurinol attenuates the development of diabetic cardiomyopathy. J Cell Mol Med 13:2330–2341
Romagnoli M, Gomez-Cabrera MC, Perrelli MG, Biasi F, Pallardó FV, Sastre J, Poli G, Viña J (2010) Xanthine oxidase-induced oxidative stress causes activation of NF-kappaB and inflammation in the liver of type I diabetic rats. Free Radic Biol Med 49:171–177
Circu ML, Aw TY (2010) Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 48:749–762
Tabak O, Gelisgen R, Erman H, Erdenen F, Muderrisoglu C, Aral H, Uzun H (2011) Oxidative lipid, protein, and DNA damage as oxidative stress markers in vascular complications of diabetes mellitus. Clin Invest Med 34:E163–E171
Sun L, Hu W, Liu Q, Hao Q, Sun B, Zhang Q, Mao S, Qiao J, Yan X (2012) Metabonomics reveals plasma metabolic changes and inflammatory marker in polycystic ovary syndrome patients. J Proteome Res 11:2937–2946
Elmarakby AA, Sullivan JC (2012) Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc Ther 30:49–59
Morgan MJ, Liu ZG (2011) Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res 21:103–115
Kosugi T, Nakayama T, Heinig M, Zhang L, Yuzawa Y, Sanchez-Lozada LG, Roncal C, Johnson RJ, Nakagawa T (2009) Effect of lowering uric acid on renal disease in the type 2 diabetic db/db mice. Am J Physiol Ren Physiol 297:F481–F488
Baker RG, Hayden MS, Ghosh S (2011) NF-kappaB, inflammation, and metabolic disease. Cell Metab 13:11–22
Gloire G, Legrand-Poels S, Piette J (2006) NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol 72:1493–1505
Acknowledgments
The authors would like to thank Dr. Pengchi Deng in Analytical & Testing Center of Sichuan University for NMR analysis. This work was supported by grants from National Natural Science Foundation of China (31200754, 30930088) and China Postdoctoral Science Foundation (2012 M511931).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 405 kb)
Rights and permissions
About this article
Cite this article
Liu, J., Wang, C., Liu, F. et al. Metabonomics revealed xanthine oxidase-induced oxidative stress and inflammation in the pathogenesis of diabetic nephropathy. Anal Bioanal Chem 407, 2569–2579 (2015). https://doi.org/10.1007/s00216-015-8481-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8481-0