Abstract
Background
In anticipation of future studies, we examined the pharmacokinetics profile of erythropoietin (EPO) in patients undergoing cardiac surgery.
Methods
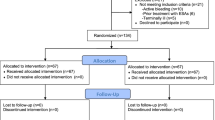
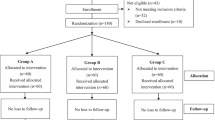
Cardiac surgical patients were enrolled into one of six groups: four cardiopulmonary bypass (CPB) groups [placebo (n = 6), 250 IU/kg EPO (n = 3), 500 IU/kg EPO (n = 3), and 500 IU/kg EPO, two doses (n = 6)] and two off-pump coronary artery bypass (OPCAB) groups [placebo (n = 3) and 500 IU/kg EPO (n = 3)]. The EPO was administered prior to anesthesia and 10 min after CPB (if required). Blood samples for serum EPO were collected at baseline, 10 min after dosing, 5 min after sternotomy, during CPB or the equivalent for OPCAB (5, 15, 45, 60 min), and post-CPB (5, 15, 45, and 60 min, 6, 12 and 24 h, and daily until day 5).
Results
Endogenous EPO increased within 24 h of surgery in the placebo group and remained elevated. There was approximately a 40% decrease in serum EPO concentration at the initiation of CPB due to an increase in circulatory blood volume. There were no differences in apparent volume of distribution in the plasma (Vc) (42.2 ± 9.9, 39.8 ± 6.3, 42.3 ± 14.0 mL/kg), clearance (CL) (4.63 ± 1.14, 3.44 ± 0.68, 4.27 ± 0.52 mL h/kg), and t½ (16.4 ± 8.0 16.9 ± 10.6, 22.4 ± 9.3 h) between the CPB treatment groups. The pharmacokinetic profile of EPO in the OPCAB group was similar to that for the CPB groups: Vc = 39.3 ± 7.0 mL/kg, CL = 4.98 ± 0.17 mL h/kg and t½ = 17.1 ± 18.1 h.
Conclusions
CPB had no apparent effect on the pharmacokinetics of EPO.



Similar content being viewed by others
Notes
http://www.fda.gov/cder/drug/infopage/RHE/default.htm; accessed 10 Oct 2007
References
Johnson RG, Thurer RL, Kruskall MS, Sirois C, Gervino EV, Critchlow J, Weintraub RM (1992) Comparison of two transfusion strategies after elective operations for myocardial revascularization. J Thorac Cardiovasc Surg 104:307–314
Goch J, Birgegard G, Wikstrom B, Backman U, Wadstrom J, Danielson BG (1996) Serum erythropoietin and erythropoiesis during six years after kidney transplantation. Nephron 74:687–693
Surgenor DM, Wallace EL, Churchill WH et al (1992) Red cell transfusions in coronary artery bypass surgery (DRGs 106 and 107). Transfusion 32:458–464
Winslow RM (1997) A physiological basis for the transfusion trigger. In: Spiess BD, Counts RB, Gould SA (eds) Perioperative transfusion medicine, 1st edn. Williams & Wilkins, Baltimore, pp 27–43
Bush RL, Pevec WC, Holcroft JW (1997) A prospective, randomized trial limiting perioperative red blood cell transfusions in vascular patients. Am J Surg 174:143–148
Cheng DCH, Karski J, Peniston C et al (1996) Morbidity outcome in early versus conventional tracheal extubation after coronary artery bypass grafting: a prospective randomized controlled trial. J Thorac Cardiovasc Surg 112:755–764
Djaiani G, Fedorko L, Cusimano RJ, Mikulis D, Carroll J, Poonawala H, Beattie S, Karski J (2006) Off-pump coronary bypass surgery: risk of ischemic brain lesions in patients with atheromatous thoracic aorta. Can J Anaesth 53(8):795–801, Aug
Quirt I, Micucci S, Moran LA, Pater J, Browman G (1997) Erythropoietin in the management of patients with nonhematologic cancer receiving chemotherapy. Cancer Prev Control 1:241–247
McMahon FG, Vargas R, Ryan M et al (1990) Pharmacokinetics and effects of recombinant human erythropoietin after intravenous and subcutaneous injections in healthy volunteers. Blood 76:1718–1722
Karkouti K, McCluskey SA, Ghannam M, Salpeter MJ, Quirt I, Yau TM (2006) Intravenous iron and recombinant erythropoietin for the treatment of postoperative anemia. Can J Anaesth 53(1):11–19, Jan
Madi-Jebara SN, Sleilaty GS, Achouh PE, Yazigi AG, Haddad FA, Hayek GM, Antakly MC, Jebara VA (2004) Postoperative intravenous iron used alone or in combination with low-dose erythropoietin is not effective for correction of anemia after cardiac surgery. J Cardiothorac Vasc Anesth 18(1):59–63
Xenocostas A, Cheung WK, Farrell F, Zakszewski C, Kelley M, Lutynski A, Crump M, Lipton JH, Kiss TL, Lau CY et al (2005) The pharmacokinetics of erythropoietin in the cerebrospinal fluid after intravenous administration of recombinant human erythropoietin. Eur J Clin Pharmacol 61(3):189–195,
Digicaylioglu M, Lipton SA (2001) Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature 412(6847):641–647, Aug 9
Dawson TM (2002) Preconditioning-mediated neuroprotection through erythropoietin? Lancet 359(9301):96–97
Lipton SA (2004) Erythropoietin for neurologic protection and diabetic neuropathy. N Engl J Med 350(24):2516–2517
Roberts WA, Kirkley SA, Newby M (1996) A cost comparison of allogeneic and preoperatively or intraoperatively donated autologous blood. Anesth Analg 83:129–133
Corwin HL, Gettinger A, Fabian TC, May A, Pearl RG, Heard S, An R, Bowers PJ, Burton P, Klausner MA (2007) Efficacy and safety of epoetin alfa in critically ill patients. N Engl J Med 357(10):965–976
Acknowledgements
This work would not have been possible without the dedication and organization of Jo Carroll, Manager Clinical Research (University Health Network, University of Toronto, Toronto, ONT, Canada).
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by an unrestricted educational grant from Ortho Biotech Canada, a Division of Janssen Ortho Inc. Preliminary data was presented at the Canadian Anesthesiology Society Meeting in June 2004.
Rights and permissions
About this article
Cite this article
McCluskey, S.A., Cheung, W.K., Katznelson, R. et al. The pharmacokinetic profile of recombinant human erythropoietin is unchanged in patients undergoing cardiac surgery. Eur J Clin Pharmacol 65, 273–279 (2009). https://doi.org/10.1007/s00228-008-0575-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-008-0575-6