Abstract
Background
Genetic polymorphisms of cytochrome P450 enzymes, especially CYP2C19, could influence voriconazole pharmacokinetics. The association between CYP2C19 polymorphisms and voriconazole clinical outcomes is not well established. The aim of this meta-analysis was to evaluate the effect of CYP2C19 polymorphisms on clinical outcomes in patients treated with voriconazole.
Methods
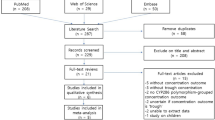
PubMed, EMBASE, CENTRAL, ClinicalTrials.gov, and three Chinese databases were searched from their inception to January 2016 to identify eligible trials that reported voriconazole exposure and clinical outcomes of voriconazole according to CYP2C19 polymorphisms. Two reviewers independently reviewed the citations, extracted the data, and assessed the quality of the trials. The meta-analysis was performed using RevMan5.3.
Results
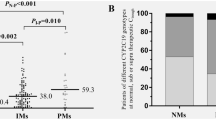
A total of ten studies involving 598 patients were included. Compared with patients with extensive metabolizer (EM) phenotype, patients with poor metabolizer (PM) phenotype had significantly higher trough concentrations (MD, 1.22 mg/L; 95 % confidence interval (CI), 0.72–1.71; P < 0.0001). PM phenotype was also associated with a higher treatment success rate compared with EM phenotype (risk ratio (RR), 1.31; 95 % CI, 1.04–1.67; P = 0.02). However, there was no significant association between CYP2C19 polymorphisms and daily maintenance dose, overall adverse events, hepatotoxicity, and neurotoxicity.
Conclusions
Patients with CYP2C19 PM phenotype were associated with increased treatment success rate and trough concentrations as compared with those with EM phenotype. There was no significant association between CYP2C19 polymorphisms and either daily maintenance dose or adverse outcomes of voriconazole. However, large-scale, high-quality trials are still needed to confirm these findings.




Similar content being viewed by others
References
Jeu L, Piacenti FJ, Lyakhovetskiy AG, Fung HB (2003) Voriconazole. Clin Ther 25:1321–1381
Hyland R, Jones BC, Smith DA (2003) Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab Dispos 31:540–547
Dolton MJ, Mikus G, Weiss J, Ray JE, McLachlan AJ (2014) Understanding variability with voriconazole using a population pharmacokinetic approach: implications for optimal dosing. J Antimicrob Chemoth 69:1633–1641
Jin H, Wang T, Falcione BA, Olsen KM, Chen K, Tang H, Hui J, Zhai S (2016) Trough concentration of voriconazole and its relationship with efficacy and safety: a systematic review and meta-analysis. J Antimicrob Chemother. doi:10.1093/jac/dkw045
Karthaus M, Lehrnbecher T, Lipp HP, Kluge S, Buchheidt D (2015) Therapeutic drug monitoring in the treatment of invasive aspergillosis with voriconazole in cancer patients--an evidence-based approach. Ann Hematol 94:547–556
de Morais SM, Wilkinson GR, Blaisdell J, Nakamura K, Meyer UA, Goldstein JA (1994) The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem 269:15419–15422
Sim SC, Risinger C, Dahl ML, Aklillu E, Christensen M, Bertilsson L, Ingelman-Sundberg M (2006) A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther 79:103–113
Pestka EL, Hale AM, Johnson BL, Lee JL, Poppe KA (2007) Cytochrome P450 testing for better psychiatric care. J Psychosoc Nurs Ment Health Serv 45:15–18
Bertilsson L (2007) Metabolism of antidepressant and neuroleptic drugs by cytochrome p450s: clinical and interethnic aspects. Clin Pharmacol Ther 82:606–609
Desta Z, Zhao X, Shin JG, Flockhart DA (2002) Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet 41:913–958
Owusu Obeng A, Egelund EF, Alsultan A, Peloquin CA, Johnson JA (2014) CYP2C19 polymorphisms and therapeutic drug monitoring of voriconazole: are we ready for clinical implementation of pharmacogenomics? Pharmacotherapy 34:703–718
Moriyama B, Kadri S, Henning SA, Danner RL, Walsh TJ, Penzak SR (2015) Therapeutic drug monitoring and genotypic screening in the clinical use of voriconazole. Curr Fungal Infect Rep 9:74–87
Wang LJ, Tang HL, Duan JL (2011) Systematic review of influence on pharmacokinetics of voriconazole on CYP2C19 genetic polymorphisms. Chin J Clin Pharmacol 27:607–611
Li XF, Yu CY, Cheng Y, Niu TW, Chen K, Tang HL (2016) Effect of cytochrome P450 2C19 genotype on pharmacokinetics of vorivonazole in healthy volunteers: a systematic review and meta-analysis. Chin J Clin Pharmacol 32:267–269
Ikeda Y, Umemura K, Kondo K, Sekiguchi K, Miyoshi S, Nakashima M (2004) Pharmacokinetics of voriconazole and cytochrome P450 2C19 genetic status. Clin Pharmacol Ther 75:587–588
Wang G, Lei HP, Li Z, Tan ZR, Guo D, Fan L, Chen Y, Hu DL, Wang D, Zhou HH (2009) The CYP2C19 ultra-rapid metabolizer genotype influences the pharmacokinetics of voriconazole in healthy male volunteers. Eur J Clin Pharmacol 65:281–285
Weiss J, Ten Hoevel MM, Burhenne J, Walter-Sack I, Hoffmann MM, Rengelshausen J, Haefeli WE, Mikus G (2009) CYP2C19 genotype is a major factor contributing to the highly variable pharmacokinetics of voriconazole. J Clin Pharmacol 49:196–204
Shi HY, Yan J, Zhu WH, Yang GP, Tan ZR, Wu WH, Zhou G, Chen XP, Ouyang DS (2010) Effects of erythromycin on voriconazole pharmacokinetics and association with CYP2C19 polymorphism. Eur J Clin Pharmacol 66:1131–1136
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13
Tang H, Yan Y, Wang T, Zhang T, Shi W, Fan R, Yao Y, Zhai S (2015) Effect of follicle-stimulating hormone receptor Asn680Ser polymorphism on the outcomes of controlled ovarian hyperstimulation: an updated meta-analysis of 16 cohort studies. J Assist Reprod Genet 32:1801–1810
Matsumoto K, Ikawa K, Abematsu K, Fukunaga N, Nishida K, Fukamizu T, Shimodozono Y, Morikawa N, Takeda Y, Yamada K (2009) Correlation between voriconazole trough plasma concentration and hepatotoxicity in patients with different CYP2C19 genotypes. Int J Antimicrob Agents 34:91–94
Bruggemann RJ, Blijlevens NM, Burger DM, Franke B, Troke PF, Donnelly JP (2010) Pharmacokinetics and safety of 14 days intravenous voriconazole in allogeneic haematopoietic stem cell transplant recipients. J Antimicrob Chemother 65:107–113
Berge M, Guillemain R, Tregouet DA, Amrein C, Boussaud V, Chevalier P, Lillo-Lelouet A, Le Beller C, Laurent-Puig P, Beaune PH, Billaud EM, Loriot MA (2011) Effect of cytochrome P450 2C19 genotype on voriconazole exposure in cystic fibrosis lung transplant patients. Eur J Clin Pharmacol 67:253–260
Kim SH, Yim DS, Choi SM, Kwon JC, Han S, Lee DG, Park C, Kwon EY, Park SH, Choi JH, Yoo JH (2011) Voriconazole-related severe adverse events: clinical application of therapeutic drug monitoring in Korean patients. Int J Infect Dis 15:e753–e758
Fu SS, Xiong X, Duan JL, Wang LJ, Liu Y, Jing HM, Zhai SD (2013) Voriconazole plasma concentration monitoring in patients. Chin J Clin Pharmacol 29:622–624
Kim SH, Lee DG, Kwon JC, Lee HJ, Cho SY, Park C, Kwon EY, Park SH, Choi SM, Choi JH, Yoo JH (2013) Clinical impact of cytochrome P450 2C19 genotype on the treatment of invasive aspergillosis under routine therapeutic drug monitoring of voriconazole in a Korean population. Infect Chemother 45:406–414
Wang T, Zhu H, Sun J, Cheng X, Xie J, Dong H, Chen L, Wang X, Xing J, Dong Y (2014) Efficacy and safety of voriconazole and CYP2C19 polymorphism for optimised dosage regimens in patients with invasive fungal infections. Int J Antimicrob Agents 44:436–442
Zonios D, Yamazaki H, Murayama N, Natarajan V, Palmore T, Childs R, Skinner J, Bennett JE (2014) Voriconazole metabolism, toxicity, and the effect of cytochrome P450 2C19 genotype. J Infect Dis 209:1941–1948
Liang FH, Meng DM, Xie H, Xiao XL, Lv BJ, Chen WY (2015) CYP2C19 gene polymorphism on plasma concentration of voriconazole in critically ill patients with invasive fungal infections. Chin J Hosp Pharm 35:1456–1461
Sumonrat C, Jantararoungtong T, Chitasombat MN, Puangpetch A, Prommas S, Dilokpattanamongkol P, Watcharananan SP, Sukasem C (2016) A prospective observational study of CYP2C19 polymorphisms and voriconazole plasma level in adult Thai patients with invasive aspergillosis. Drug Metab Pharmacokinet 31:117–122
Ueda K, Nannya Y, Kumano K, Hangaishi A, Takahashi T, Imai Y, Kurokawa M (2009) Monitoring trough concentration of voriconazole is important to ensure successful antifungal therapy and to avoid hepatic damage in patients with hematological disorders. Int J Hematol 89:592–599
Levin MD, den Hollander JG, van der Holt B, Rijnders BJ, van Vliet M, Sonneveld P, van Schaik RH (2007) Hepatotoxicity of oral and intravenous voriconazole in relation to cytochrome P450 polymorphisms. J Antimicrob Chemother 60:1104–1107
Acknowledgments
The authors would like to thank Drs Dong and Bruggemann for providing the requested data. We also thank Dr Falcione Bonnie who provided language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Li, X., Yu, C., Wang, T. et al. Effect of cytochrome P450 2C19 polymorphisms on the clinical outcomes of voriconazole: a systematic review and meta-analysis. Eur J Clin Pharmacol 72, 1185–1193 (2016). https://doi.org/10.1007/s00228-016-2089-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-016-2089-y