Abstract
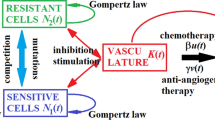
A minimally parameterized mathematical model for low-dose metronomic chemotherapy is formulated that takes into account angiogenic signaling between the tumor and its vasculature and tumor inhibiting effects of tumor-immune system interactions. The dynamical equations combine a model for tumor development under angiogenic signaling formulated by Hahnfeldt et al. with a model for tumor-immune system interactions by Stepanova. The dynamical properties of the model are analyzed. Depending on the parameter values, the system encompasses a variety of medically realistic scenarios that range from cases when (i) low-dose metronomic chemotherapy is able to eradicate the tumor (all trajectories converge to a tumor-free equilibrium point) to situations when (ii) tumor dormancy is induced (a unique, globally asymptotically stable benign equilibrium point exists) to (iii) multi-stable situations that have both persistent benign and malignant behaviors separated by the stable manifold of an unstable equilibrium point and finally to (iv) situations when tumor growth cannot be overcome by low-dose metronomic chemotherapy. The model forms a basis for a more general study of chemotherapy when the main components of a tumor’s microenvironment are taken into account.






Similar content being viewed by others
References
André N, Padovani L, Pasquier E (2011) Metronomic scheduling of anticancer treatment: the next generation of multitarget therapy? Future Oncol 7(3):385–394
Benzekry S, Hahnfeldt P (2013) Maximum tolerated dose versus metronomic scheduling in the treatment of metastatic cancers. J Theor Biol 335:235–244
Benzekry S, André N, Benabdallah A, Ciccolini J, Faivre C, Hubert F, Barbolosi D (2012) Modeling the impact of anticancer agents on metastatic spreading. Math Model Nat Phenom 7(1):306–336. doi:10.1051/mmnp/20127114
Bocci G, Nicolaou K, Kerbel RS (2002) Protracted low-dose effects on human endothelial cell proliferation and survival in vitro reveal a selective antiangiogenic window for various chemotherapeutic drugs. Cancer Res 62:6938–6943
Browder T, Butterfield CE, Kräling BM, Shi B, Marshall B, O’Reilly MS, Folkman J (2000) Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res 60:1878–1886
d’Onofrio A (2005) A general framework for modelling tumor-immune system competition and immunotherapy: mathematical analysis and biomedical inferences. Physica D 208:202–235
d’Onofrio A (2006) Tumor-immune system interaction: modeling the tumor-stimulated proliferation of effectors and immunotherapy. Math Models Methods Appl Sci 16:1375–1401
d’Onofrio A (2009) Fractal growth of tumors and other cellular populations: linking the mechanistic to the phenomenological modeling and vice versa. Chaos Solitons Fractals 41:875–880
d’Onofrio A, Gandolfi A (2004) Tumour eradication by antiangiogenic therapy: analysis and extensions of the model by Hahnfeldt et al. Math. Biosci. 191:159–184
d’Onofrio A, Gandolfi A (2009) A family of models of angiogenesis and anti-angiogenesis anti-cancer therapy. Math Med Biol 26:63–95. doi:10.1093/imammb/dqn024
d’Onofrio A, Ledzewicz U, Maurer H, Schättler H (2009) On optimal delivery of combination therapy for tumors. Math Biosci 222:13–26. doi:10.1016/j.mbs.2009.08.004
d’Onofrio A, Ledzewicz U, Schättler H (2012) On the dynamics of tumor-immune system interactions and combined chemo- and immunotherapy. In: d’Onofrio A, Cerrai P, Gandolfi A (eds) New challenges for cancer systems biomedicine. SIMAI Springer Series, vol 1. Springer, Milan, pp 249–266
Davis S, Yancopoulos GD (1999) The angiopoietins: Yin and Yang in angiogenesis. Curr Top Microbiol Immunol 237:173–185
de Pillis LG, Radunskaya A, Wiseman CL (2005) A validated mathematical model of cell-mediated immune response to tumor growth. Cancer Res 65:7950–7958
de Pillis LG, Mallet DG, Radunskaya AE (2006) Spatial tumor-immune modeling, special issue devoted to cancer and medical treatment modelling. J Comput Math Methods Med 7:159–176
de Vladar HP, González JA (2004) Dynamic response of cancer under the influence of immunological activity and therapy. J Theor Biol 227:335–348
Delitalia M, Lorenzi T (2013) Recognition and learning in a mathematical model for immune response against cancer. Discret Top Contin Dyn Syst Ser B 18:891–914
Eftimie R, Bramson JL, Earn DJD (2010) Interactions between the immune system and cancer: a brief review of non-spatial mathematical models. Bull Math Biol 73(1):2–32
Ergun A, Camphausen K, Wein LM (2003) Optimal scheduling of radiotherapy and angiogenic inhibitors. Bull Math Biol 65:407–424
Friedman A, Kim Y (2011) Tumor cell proliferation and migration under the influence of their microenvironment. Math Biosci Eng 8(2):371–383
Gatenby RA, Silva AS, Gillies RJ, Frieden BR (2009) Adaptive therapy. Cancer Res 69:4894–4903
Guckenheimer J, Holmes P (1983) Nonlinear oscillations, dynamical systems, and bifurcations of vector fields. Springer, New York
Hahnfeldt P, Panigrahy D, Folkman J, Hlatky L (1999) Tumor development under angiogenic signaling: a dynamical theory of tumor growth, treatment response, and postvascular dormancy. Cancer Res 59:4770–4775
Hanahan D, Bergers G, Bergsland E (2000) Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Investig 105(8):1045–1047
Hahnfeldt P, Folkman J, Hlatky L (2003) Minimizing long-term burden: the logic for metronomic chemotherapeutic dosing and its angiogenic basis. J Theor Biol 220:545–554
Kamen B, Rubin E, Aisner J, Glatstein E (2000) High-time chemotherapy or high time for low dose? J Clin Oncol 18:2935–2937 (editorial)
Kim Y, Friedman A (2010) Interaction of tumor with its microenvironment: a mathematical model. Bull Math Biol 72:1029–1068
Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS (2000) Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J. Clin Investig 105(8):R15–R24
Kuznetsov VA, Makalkin IA, Taylor MA, Perelson AS (1994) Nonlinear dynamics of immunogenic tumors: parameter estimation and global bifurcation analysis. Bull Math Biol 56:295–321
Lavi O, Greene JM, Levy D, Gottesman MM (2013) The role of cell density and intratumoral heterogeneity in multidrug resistance. Cancer Res 73(24):7168–7175
Ledzewicz U, Schättler H (2007) Anti-angiogenic therapy in cancer treatment as an optimal control problem. SIAM J Control Optim 46:1052–1079
Ledzewicz U, Schättler H (2014a) A review of optimal chemotherapy protocols: from MTD towards metronomic therapy. Math Model Nat Phenom 9(4):131–152. doi:10.1051/mmnp/20149409
Ledzewicz U, Schättler H (2014b) Tumor microenvironment and anticancer therapies: an optimal control approach. In: d’Onofrio A, Gandolfi A (eds) Mathematical oncology. Springer, Berlin
Ledzewicz U, Naghnaeian M, Schättler H (2012a) Optimal response to chemotherapy for a mathematical model of tumor-immune dynamics. J Math Biol 64:557–577. doi:10.1007/s00285-011-0424-6
Ledzewicz U, Olumoye O, Schättler H (2012b) On optimal chemotherapy with a stongly targeted agent for a model of tumor-immune system interactions with generalized logistic growth. Math Biosci Eng 10(3):787–802. doi:10.3934/mbe.2013.10.787
Ledzewicz U, Faraji Mosalman MS, Schättler H (2013) Optimal controls for a mathematical model of tumor-immune interactions under targeted chemotherapy with immune boost. Discret Contin Dyn Syst Ser B 18:1031–1051. doi:10.3934/dcdsb.2013.18.1031
Lorz A, Lorenzi T, Hochberg ME, Clairambault J, Perthame B (2013) Populational adaptive evolution, chemotherapeutic resistance and multiple anti-cancer therapies. ESAIM Math Model Numer Anal 47:377–399. doi:10.1051/m2an/2012031
Norton L, Simon R (1977) Tumor size, sensitivity to therapy, and design of treatment schedules. Cancer Treat Rep 61:1307–1317
Norton L, Simon R (1986) The Norton–Simon hypothesis revisited. Cancer Treat Rep 70:41–61
Pasquier E, Ledzewicz U (2013) Perspective on ”More is not necessarily better”: metronomic chemotherapy. Newslett Soc Math Biol 26(2):9–10
Pasquier E, Kavallaris M, André N (2010) Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol 7:455–465
Pietras K, Hanahan D (2005) A multi-targeted, metronomic and maximum tolerated dose ”chemo-switch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J Clin Oncol 23:939–952
Schättler H, Ledzewicz U, Cardwell B (2011) Robustness of optimal controls for a class of mathematical models for tumor anti-angiogenesis. Math Biosci Eng 8(2):355–369
Skipper HE (1986) On mathematical modeling of critical variables in cancer treatment (goals: better understanding of the past and better planning in the future). Bull Math Biol 48:253–278
Sole RV (2003) Phase transitions in unstable cancer cell populations. Eur J Phys B 35:117–124
Stepanova NV (1980) Course of the immune reaction during the development of a malignant tumour. Biophysics 24:917–923
Swann JB, Smyth MJ (2007) Immune surveillance of tumors. J Clin Investig 117:1137–1146
Weitman SD, Glatstein E, Kamen BA (1993) Back to the basics: the importance of concentration \(\times \) time in oncology. J Clin Oncol 11:820–821
Wheldon TE (1988) Mathematical models in cancer research. Hilger Publishing, Boston
Whiteside TL (2008) The tumor microenvironment and its role in promoting tumor growth. Oncogene 27:5904–5912
Acknowledgments
This material is based upon work supported by the National Science Foundation under collaborative research Grants Nos. DMS 1311729/1311733. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schättler, H., Ledzewicz, U. & Amini, B. Dynamical properties of a minimally parameterized mathematical model for metronomic chemotherapy. J. Math. Biol. 72, 1255–1280 (2016). https://doi.org/10.1007/s00285-015-0907-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-015-0907-y
Keywords
- Dynamical system
- Saddle-node bifurcations
- Modeling of cancer treatment
- Metronomic chemotherapy
- Tumor microenvironment