Abstract
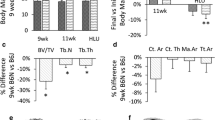
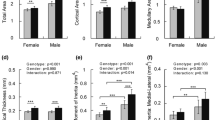
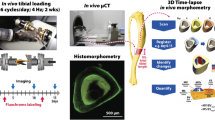
Biglycan (bgn)-deficient mice (KO) have defective osteoblasts which lead to changes in the amount and quality of bone. Altered tissue strength in C57BL6/129 (B6;129) KO mice, a property which is independent of tissue quantity, suggests that deficiencies in tissue quality are responsible. However, the response to bgn-deficiency is inbred strain-specific. Mechanical loading influences bone matrix quality in addition to any increase in bone mass or change in bone formation activity. Since many diseases influence the mechanical integrity of bone through altered tissue quality, loading may be a way to prevent and treat extracellular matrix deficiencies. C3H/He (C3H) mice consistently have a less vigorous response to mechanical loading vs. other inbred strains. It was therefore hypothesized that the bones from both wild type (WT) and KO B6;129 mice would be more responsive to exercise than the bones from C3H mice. To test these hypotheses at 11 weeks of age, following 21 consecutive days of exercise, we investigated cross-sectional geometry, mechanical properties, and tissue composition in the tibiae of male mice bred on B6;129 and C3H backgrounds. This study demonstrated inbred strain-specific compositional and mechanical changes following exercise in WT and KO mice, and showed evidence of genotype-specific changes in bone in response to loading in a gene disruption model. This study further shows that exercise can influence bone tissue composition and/or mechanical integrity without changes in bone geometry. Together, these data suggest that exercise may represent a possible means to alter tissue quality and mechanical deficiencies caused by many diseases of bone.




Similar content being viewed by others
References
Adami, S., D. Gatti, V. Braga, D. Bianchini, and M. Rossini. Site-specific effects of strength training on bone structure and geometry of ultradistal radius in postmenopausal women. J. Bone Miner. Res. 14:120–124, 1999.
Akhter, M. P., D. M. Cullen, E. A. Pedersen, D. B. Kimmel, and R. R. Recker. Bone response to in vivo mechanical loading in two breeds of mice. Calcif. Tissue Int. 63:442–449, 1998.
Amblard, D., M. H. Lafage-Proust, A. Laib, T. Thomas, P. Ruegsegger, C. Alexandre, and L. Vico. Tail suspension induces bone loss in skeletally mature mice in the C57BL/6J strain but not in the C3H/HeJ strain. J. Bone Miner. Res. 18:561–569, 2003.
Ameye, L., D. Aria, K. Jepsen, A. Oldberg, T. Xu, and M. F. Young. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice leads to gait impairment, ectopic ossification, and osteoarthritis. FASEB J. 16:673–680, 2002.
Baig, A., J. Fox, R. Young, Z. Wang, J. Hsu, W. Higuchi, A. Chhettry, H. Zhuang, and M. Otsuka. Relationships among carbonated apatite solubility, crystallite size, and microstrain parameters. Calcif. Tissue Int. 64:437–449, 1999.
Bhowmik, R., K. S. Katti, and D. R. Katti. Mechanics of molecular collagen is influenced by hydroxyapatite in natural bone. J. Mater. Sci. 42:8795–8803, 2007.
Bianco, P., L. W. Fisher, M. F. Young, J. D. Termine, and P. G. Robey. Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues. J. Histochem. Cytochem. 38:1549–1563, 1990.
Boskey, A. L., T. M. Wright, and R. D. Blank. Collagen and bone strength. J. Bone Miner. Res. 14:330–335, 1999.
Burger, E. H., and J. Klein-Nulend. Mechanotransduction in bone–role of the lacuno-canalicular network. FASEB J. 13(Suppl):S101–S112, 1999.
Burstein, A. H., J. M. Zika, K. G. Heiple, and L. Klein. Contribution of collagen and mineral to the elastic-plastic properties of bone. J. Bone Joint Surg. Am. 57:956–961, 1975.
Carden, A., R. M. Rajachar, M. D. Morris, and D. H. Kohn. Ultrastructural changes accompanying the mechanical deformation of bone tissue: a Raman imaging study. Calcif. Tissue Int. 72:166–175, 2003.
Chen, X. D., M. R. Allen, S. Bloomfield, T. Xu, and M. Young. Biglycan-deficient mice have delayed osteogenesis after marrow ablation. Calcif. Tissue Int. 72:577–582, 2003.
Chen, X. D., L. W. Fisher, P. G. Robey, and M. F. Young. The small leucine-rich proteoglycan biglycan modulates BMP-4-induced osteoblast differentiation. FASEB J. 18:948–958, 2004.
Chen, X. D., S. Shi, T. Xu, P. G. Robey, and M. F. Young. Age-related osteoporosis in biglycan-deficient mice is related to defects in bone marrow stromal cells. J. Bone Miner. Res. 17:331–340, 2002.
Corsi, A., T. Xu, X. D. Chen, A. Boyde, J. Liang, M. Mankani, B. Sommer, R. V. Iozzo, I. Eichstetter, P. G. Robey, P. Bianco, and M. F. Young. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J. Bone Miner. Res. 17:1180–1189, 2002.
Duncan, R. L., and C. H. Turner. Mechanotransduction and the functional response of bone to mechanical strain. Calcif. Tissue Int. 57:344–358, 1995.
Follet, H., G. Boivin, C. Rumelhart, and P. J. Meunier. The degree of mineralization is a determinant of bone strength: a study on human calcanei. Bone 34:783–789, 2004.
Fritsch, A., C. Hellmich, and L. Dormieux. Ductile sliding between mineral crystals followed by rupture of collagen crosslinks: experimentally supported micromechanical explanation of bone strength. J. Theor. Biol. 260:230–252, 2009.
Hoshi, A., H. Watanabe, M. Chiba, and Y. Inaba. Bone density and mechanical properties in femoral bone of swim loaded aged mice. Biomed. Environ. Sci. 11:243–250, 1998.
Isaksson, H., V. Tolvanen, M. A. J. Finnilä, J. Iivarinen, J. Tuukkanen, K. Seppänen, J. P. A. Arokoski, P. A. Brama, J. S. Jurvelin, and H. J. Helminen. Physical exercise improves properties of bone and its collagen network in growing and maturing mice. Calcif. Tissue Int. 85(3):247–256, 2009.
Kodama, Y., Y. Umemura, S. Nagasawa, W. G. Beamer, L. R. Donahue, C. R. Rosen, D. J. Baylink, and J. R. Farley. Exercise and mechanical loading increase periosteal bone formation and whole bone strength in C57BL/6J mice but not in C3H/Hej mice. Calcif. Tissue Int. 66:298–306, 2000.
Kohn, D. H., N. D. Sahar, J. M. Wallace, K. Golcuk, and M. D. Morris. Exercise alters mineral and matrix composition in the absence of adding new bone. Cells Tissues Organs 189:33–37, 2009.
Kuhn, J. L., S. A. Goldstein, L. A. Feldkamp, R. W. Goulet, and G. Jesion. Evaluation of a microcomputed tomography system to study trabecular bone structure. J. Orthop. Res. 8:833–842, 1990.
Landis, W. J. The strength of a calcified tissue depends in part on the molecular structure and organization of its constituent mineral crystals in their organic matrix. Bone 16:533–544, 1995.
Li, X., W. Gu, G. Masinde, M. Hamilton-Ulland, C. H. Rundle, S. Mohan, and D. J. Baylink. Genetic variation in bone-regenerative capacity among inbred strains of mice. Bone 29:134–140, 2001.
Marusic, A., V. Katavic, D. Grcevic, and I. K. Lukic. Genetic variability of new bone induction in mice. Bone 25:25–32, 1999.
Miller, L. M., W. Little, A. Schirmer, F. Sheik, B. Busa, and S. Judex. Accretion of bone quantity and quality in the developing mouse skeleton. J. Bone Miner. Res. 22:1037–1045, 2007.
Mosekilde, L., J. S. Thomsen, P. B. Orhii, R. J. McCarter, W. Mejia, and D. N. Kalu. Additive effect of voluntary exercise and growth hormone treatment on bone strength assessed at four different skeletal sites in an aged rat model. Bone 24:71–80, 1999.
Paschalis, E. P., K. Verdelis, S. B. Doty, A. L. Boskey, R. Mendelsohn, and M. Yamauchi. Spectroscopic characterization of collagen cross-links in bone. J. Bone Miner. Res. 16:1821–1828, 2001.
Robling, A. G., F. M. Hinant, D. B. Burr, and C. H. Turner. Shorter, more frequent mechanical loading sessions enhance bone mass. Med. Sci. Sports Exerc. 34:196–202, 2002.
Takagi, M., T. Yamada, N. Kamiya, T. Kumagai, and A. Yamaguchi. Effects of bone morphogenetic protein-2 and transforming growth factor-beta1 on gene expression of decorin and biglycan by cultured osteoblastic cells. Histochem. J. 31:403–409, 1999.
Teti, A., and A. Zallone. Do osteocytes contribute to bone mineral homeostasis? Osteocytic osteolysis revisited. Bone 44:11–16, 2009.
Timlin, J. A., A. Carden, M. D. Morris, R. M. Rajachar, and D. H. Kohn. Raman spectroscopic imaging markers for fatigue-related microdamage in bovine bone. Anal. Chem. 72:2229–2236, 2000.
Turner, C. H., and D. B. Burr. Basic biomechanical measurements of bone: a tutorial. Bone 14:595–607, 1993.
Vaidya, S., C. Karunakaran, B. Pande, N. Gupta, R. Iyer, and S. Karweer. Pressure-induced crystalline to amorphous transition in hydroxylapatite. J. Mater. Sci. 32:3213–3217, 1997.
Wallace, J. M., K. Golcuk, M. D. Morris, and D. H. Kohn. Inbred strain-specific response to biglycan deficiency in the cortical bone of C57BL6/129 and C3H/He mice. J. Bone Miner. Res. 24:1002–1012, 2009.
Wallace, J. M., R. M. Rajachar, M. R. Allen, S. A. Bloomfield, P. G. Robey, M. F. Young, and D. H. Kohn. Exercise-induced changes in the cortical bone of growing mice are bone- and gender-specific. Bone 40:1120–1127, 2007.
Wallace, J. M., R. M. Rajachar, X. D. Chen, S. Shi, M. R. Allen, S. A. Bloomfield, C. M. Les, P. G. Robey, M. F. Young, and D. H. Kohn. The mechanical phenotype of biglycan-deficient mice is bone- and gender-specific. Bone 39:106–116, 2006.
Wallace, J. M., M. S. Ron, and D. H. Kohn. Short-term exercise in mice increases tibial post-yield mechanical properties while two weeks of latency following exercise increases tissue-level strength. Calcif. Tissue Int. 84:297–304, 2009.
Wang, X., X. Li, R. A. Bank, and C. M. Agrawal. Effects of collagen unwinding and cleavage on the mechanical integrity of the collagen network in bone. Calcif. Tissue Int. 71:186–192, 2002.
Wang, X., X. Shen, X. Li, and C. M. Agrawal. Age-related changes in the collagen network and toughness of bone. Bone 31:1–7, 2002.
Weiner, S., and W. Traub. Bone structure: from angstroms to microns. FASEB J. 6:879–885, 1992.
Wilson, E. E., A. Awonusi, M. D. Morris, D. H. Kohn, M. M. Tecklenburg, and L. W. Beck. Three structural roles for water in bone observed by solid-state NMR. Biophys. J. 90:3722–3731, 2006.
Wilson, E. E., A. Awonusi, M. D. Morris, D. H. Kohn, M. M. Tecklenburg, and L. W. Beck. Highly ordered interstitial water observed in bone by nuclear magnetic resonance. J. Bone Miner. Res. 20:625–634, 2005.
Wolff, J., P. Maquet, and R. Furlong. The Law of Bone Remodelling. Berlin, New York: Springer-Verlag Berlin and Heidelberg GmbH & Co. K, p. 126, 1986.
Xu, T., P. Bianco, L. W. Fisher, G. Longenecker, E. Smith, S. Goldstein, J. Bonadio, A. Boskey, A. M. Heegaard, B. Sommer, K. Satomura, P. Dominguez, C. Zhao, A. B. Kulkarni, P. G. Robey, and M. F. Young. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat. Genet. 20:78–82, 1998.
Yeh, J. K., J. F. Aloia, and S. Yasumura. Effect of physical activity on calcium and phosphorus metabolism in the rat. Am. J. Physiol. 256:E1–E6, 1989.
Acknowledgments
Funding Sources: DoD/US Army DAMD17-03-1-0556; NIH R01 AR050210; NIH P30-AR46024; NIH IPA Agreement; NIH Regenerative Sciences Training Grant R90-DK071506.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Eric M. Darling oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Wallace, J.M., Golcuk, K., Morris, M.D. et al. Inbred Strain-Specific Effects of Exercise in Wild Type and Biglycan Deficient Mice. Ann Biomed Eng 38, 1607–1617 (2010). https://doi.org/10.1007/s10439-009-9881-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-009-9881-0