Abstract
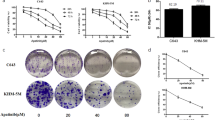
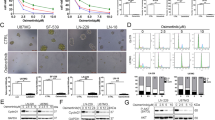
Mutations in REarranged during Transfection (RET) receptor tyrosine, followed by the oncogenic activation of RET kinase is responsible for the development of medullary thyroid carcinoma (MTC) that responds poorly to conventional chemotherapy. Targeting RET, therefore, might be useful in tailoring surveillance of MTC patients. Here we showed that theaflavins, the bioactive components of black tea, successfully induced apoptosis in human MTC cell line, TT, by inversely modulating two molecular pathways: (i) stalling PI3K/Akt/Bad pathway that resulted in mitochondrial transmembrane potential (MTP) loss, cytochrome-c release and activation of the executioner caspases-9 and -3, and (ii) upholding p38MAPK/caspase-8/caspase-3 pathway via inhibition of Ras/Raf/ERK. Over-expression of either constitutively active myristoylated-Akt-cDNA (Myr-Akt-cDNA) or dominant-negative-caspase-8-cDNA (Dn-caspase-8-cDNA) partially blocked theaflavin-induced apoptosis, while co-transfection of Myr-Akt-cDNA and Dn-caspase-8-cDNA completely eradicated the effect of theaflavins thereby negating the possibility of existence of other pathways. A search for the upstream signaling revealed that theaflavin-induced disruption of lipid raft caused interference in anchorage of RET in lipid raft that in turn stalled phosphorylation of Ras and PI3Kinase. In such anti-survival cellular micro-environment, pro-apoptotic signals were triggered to culminate into programmed death of MTC cell. These findings not only unveil a hitherto unexplained mechanism underlying theaflavin-induced MTC death, but also validate RET as a promising and potential target for MTC therapy.






Similar content being viewed by others
Abbreviations
- cDNA:
-
Complementary deoxyribonucleic acid
- ERK:
-
Extracellular signal-regulated protein kinases
- IB:
-
Immunoblotting
- IP:
-
Immunoprecipitation
- MEN 2:
-
Multiple endocrine neoplasia type 2
- MTC:
-
Medullary thyroid cancer
- p38MAPK:
-
p38 Mitogen-activated protein kinase
- RET:
-
REarranged during Transfection
- siRNA:
-
Short-interfering RNA
References
Stratakis CA, Ball DW (2000) A concise genetic and clinical guide to multiple endocrine neoplasias and related syndromes. J Pediatr Endocrinol Metab 13:457–465
Torino F, Paragliola RM, Barnabei A, Corsello SM (2010) Medullary thyroid cancer: a promising model for targeted therapy. Curr Mol Med 10:608–625
Kebebew E, Clark OH (2000) Medullary thyroid cancer. Curr Treat Options Oncol 1:359–667
Cakir M, Grossman AB (2009) Medullary thyroid cancer: molecular biology and novel molecular therapies. Neuroendocrinology 90:323–348
Wells SA Jr, Santoro M (2009) Targeting the RET pathway in thyroid cancer. Clin Caner Res 15:7119–7123
Kouvaraki MA, Shapiro SE, Perrier ND, Cote GJ, Gagel RF, Hoff AO et al (2005) RET proto-oncogene: a review and update of genotype-phenotype correlations in hereditary medullary thyroid cancer and associated endocrine tumors. Thyroid 15:531–544
Takahashi M (2001) The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev 12:361–373
Cranston A, Carniti C, Martin S, Mondellini P, Hooks Y, Leyland J et al (2006) A novel activating mutation in the RET tyrosine kinase domain mediates neoplastic transformation. Mol Endocrinol 20:1633–1643
Putzer BM, Drosten M (2004) The RET proto-oncogene: a potential target for molecular cancer therapy. Trends Mol Med 10:351–357
Ichihara M, Murakumo Y, Takahashi M (2004) RET and neuroendocrine tumors. Cancer Lett 204:197–211
Gallel P, Pallares J, Dolcet X, Llobet D, Eritja N, Santacana M et al (2008) Nuclear factor-kappaB activation is associated with somatic and germ line RET mutations in medullary thyroid carcinoma. Hum Pathol 39:994–1001
Santoro M, Carlomagno F, Melillo RM, Fusco A (2004) Dysfunction of the RET receptor in human cancer. Cell Mol Life Sci 61:2954–2964
de Groot JW, Links TP, Plukker JT, Lips CJ, Hofstra RM (2006) RET as a diagnostic and therapeutic target in sporadic and hereditary endocrine tumors. Endocinol Rev 27:535–560
Santarpia L, Ye L, Gagel RF (2009) Beyond RET: potential therapeutic approaches for advanced and metastatic medullary thyroid carcinoma. J Intern Med 266:99–113
Kuo CT, Hsu MJ, Chen BC, Chen CC, Teng CM, Pan SL et al (2008) Denbinobin induces apoptosis in human lung adenocarcinoma cells via Akt inactivation, Bad activation, and mitochondrial dysfunction. Toxicol Lett 177:48–58
Yoon CH, Kim MJ, Park MT, Byun JY, Choi YH, Yoo HS et al (2009) Acivation of p38 mitogen-activated protein kinase is required for death receptor independent caspase-8 activation and cell death in response to sphingosine. Mol Cancer Res 7:361–370
Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270:1326–1331
Yana I, Nakamura T, Shin E, Karakawa K, Kurahashi H, Kurita Y et al (1992) Inactivation of the p53 gene is not required for tumorigenesis of medullary thyroid carcinoma or pheochromocytoma. Jpn J Cacer Res 83:1113–1116
Pierchala BA, Milbrandt J, Johnson EM Jr (2006) Glial cell line-derived neurotrophic factor-dependent recruitment of Ret into lipid rafts enhances signaling by partitioning Ret from proteasome-dependent degradation. J Neurosci 26:2777–2787
Kodama Y, Asai N, Kawai K, Jijiwa M, Murakumo Y, Ichihara M et al (2005) The RET proto-oncogene: a molecular therapeutic target in thyroid cancer. Cancer Sci 96:143–148
Mologni L (2011) Development of RET kinase inhibitors for targeted cancer therapy. Curr Med Chem 18:162–175
Lahiry L, Saha B, Chakraborty J, Bhattacharyya S, Chattopadhyay S, Banerjee S et al (2008) Contribution of p53-mediated Bax transactivation in theaflavin-induced mammary epithelial carcinoma cell apoptosis. Apoptosis 13:771–781
Lahiry L, Saha B, Chakraborty J, Adhikary A, Mohanty S, Hossain DM et al (2010) Theaflavins target Fas/caspase-8 and Akt/pBad pathways to induce apoptosis in p53-mutated human breast cancer cells. Carcinogenesis 31:259–268
Adhikary A, Mohanty S, Lahiry L, Hossain DM, Chakraborty S, Das T (2010) Theaflavins retard human breast cancer cell migration by inhibiting NF-kappaB via p53-ROS cross-talk. FEBS Lett 584:7–14
Li YC, Park MJ, Ye SK, Kim CW, Kim YN (2006) Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol 168:1107–1118
Bhattacharyya A, Mandal D, Lahiry L, Bhattacharyya S, Chattopadhyay S, Ghosh UK et al (2007) Black tea-induced amelioration of hepatic oxidative stress through antioxidative activity in EAC-bearing mice. J Environ Pathol Toxicol Oncol 26:245–254
Choudhuri T, Pal S, Das T, Sa G (2005) Curcumin selectively induces apoptosis in deregulated cyclin D1-expressed cells at G2 phase of cell cycle in a p53-dependent manner. J Biol Chem 280:20059–20068
Jaqaman K, Kuwata H, Touret N, Collins R, Trimble WS, Danuser G et al (2011) Cytoskeletal control of CD36 diffusion promotes its receptor and signaling function. Cell 146:593–606
Balasubramanian N, Scott DW, Castle JD, Casanova JE, Schwartz MA (2007) Arf6 and microtubules in adhesion-dependent trafficking of lipid rafts. Nat Cell Biol 9(12):1381–1391
Chakraborty J, Banerjee S, Ray P, Hossain DM, Bhattacharyya S, Adhikary A et al (2010) Gain of cellular adaptation due to prolonged p53 impairment leads to functional switchover from p53 to p73 during DNA damage in acute myeloid leukemia cells. J Biol Chem 285:33104–33112
Stennicke HR, Jürgensmeier JM, Shin H, Deveraux Q, Wolf BB, Yang X et al (1998) Pro-caspase-3 is a major physiologic target of caspase-8. J Biol Chem 273:27084–27090
Mitsiades N, Poulaki V, Tseleni-Balafouta S, Koutras DA, Stamenkovic I (2000) Thyroid carcinoma cells are resistant to FAS-mediated apoptosis but sensitive to tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res 60:4122–4129
Nicolini V, Cassinelli G, Cuccuru G, Bongarzone I, Petrangolini G, Tortoreto M et al (2011) Interplay between Ret and Fap-1 regulates CD95-mediated apoptosis in medullary thyroid cancer cells. Biochem Pharmacol 82(7):778–788
Rzeszutko M, Rzeszutko W, Dziegiel P, Balcerzak W, Kaliszewski K, Bolanowski M (2007) Expression of FAS/APO 1/CD 95 in thyroid tumors. Folia Histochem Cytobiol 45:87–91
Park MT, Choi JA, Kim MJ, Um HD, Bae S, Kang CM et al (2003) Suppression of extracellular signal-related kinase and activation of p38 MAPK are two critical events leading to caspase-8- and mitochondria-mediated cell death in phytosphingosine-treated human cancer cells. J Biol Chem 278:50624–50634
Xiao YQ, Malcolm K, Worthen GS, Gardai S, Schiemann WP, Fadok VA et al (2002) Cross-talk between ERK and p38 MAPK mediates selective suppression of pro-inflammatory cytokines by transforming growth factor-beta. J Biol Chem 277:14884–14893
Soderstrom TS, Poukkula M, Holmstrom TH, Heiskanen KM, Eriksson JE (2002) Mitogen-activated protein kinase/extracellular signal-regulated kinase signaling in activated T cells abrogates TRAIL-induced apoptosis upstream of the mitochondrial amplification loop and caspase-8. J Immunol 169:2851–2860
Boutros T, Chevet E, Metrakos P (2008) Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol Rev 60:261–310
Peyssonnaux C, Eychène A (2001) The Raf/MEK/ERK pathway: new concepts of activation. Biol Cell 93:53–62
Giehl K, Skripczynski B, Mansard A, Menke A, Gierschik P (2000) Growth factor-dependent activation of the Ras-Raf-MEK-MAPK pathway in the human pancreatic carcinoma cell line PANC-1 carrying activated K-ras: implications for cell proliferation and cell migration. Oncogene 19:2930–2942
Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y et al (1997) Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231–241
Richardson DS, Lai AZ, Mulligan LM (2006) RET ligand-induced internalization and its consequences for downstream signaling. Oncogene 25(22):3206–3211
Shin I, Yakes FM, Rojo F, Shin NY, Bakin AV, Baselga J et al (2002) PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med 8:1145–1152
Rodríguez-Antona C, Pallares J, Montero-Conde C, Inglada-Pérez L, Castelblanco E, Landa I et al (2010) Overexpression and activation of EGFR and VEGFR2 in medullary thyroid carcinomas is related to metastasis. Endocr Relat Cancer 17(1):7–16
Hayashi H, Ichihara M, Iwashita T, Murakami H, Shimono Y, Kawai K et al (2000) Characterization of intracellular signals via tyrosine 1062 in RET activated by glial cell line-derived neurotrophic factor. Oncogene 19:4469–4475
Viglietto G, Chiappetta G, Martinez-Tello FJ, Fukunaga FH, Tallini G, Rigopoulou D et al (1995) RET/PTC oncogene activation is an early event in thyroid carcinogenesis. Oncogene 11:1207–1210
Hodgson JM, Croft KD (2010) Tea flavonoids and cardiovascular health. Mol Aspects Med 31(6):495–502
Mujtaba T, Duo QP (2012) Black tea polyphenols inhibit tumor proteasome activity. In Vivo 26(2):197–202
Wiseman S, Mulder T, Rietveld A (2001) Tea flavonoids: bioavailability in vivo and effects on cell signaling pathways in vitro. Antioxid Redox Signal 3:1009–1021
Mulder TP, van Platerink CJ, Wijnand Schuyl PJ, van Amelsvoort JM (2001) Analysis of theaflavins in biological fluids using liquid chromatography-electrospray mass spectrometry. J Chromatogr B Biomed Sci Appl 760:271–279
Henning SM, Aronson W, Niu Y, Conde F, Lee NH, Seeram NP et al (2006) Tea polyphenols and theaflavins are present in prostate tissue of humans and mice after green and black tea consumption. J Nutr 136:1839–1843
Saha B, Adhikary A, Ray P, Saha S, Chakraborty S, Mohanty S et al (2011) Restoration of tumor suppressor p53 by differentially regulating pro- and anti-p53 networks in HPV-18-infected cervical cancer cells. Oncogene 31:173–186
Herfarth KK, Wick MR, Marshall HN, Gartner E, Lum S, Moley JF (1997) Absence of TP53 alterations in pheochromocytomas and medullary thyroid carcinomas. Genes Chromosomes Cancer 20:24–29
Micheau O, Solary E, Hammann A, Dimanche-Boitrel MT (1999) Fas ligand-independent, FADD-mediated activation of the Fas death pathway by anticancer drugs. J Biol Chem 274:7987–7992
Shammas MA, Neri P, Koley H, Batchu RB, Bertheau RC, Munshi V et al (2006) Specific killing of multiple myeloma cells by (−)-epigallocatechin-3-gallate extracted from green tea: biologic activity and therapeutic implications. Blood 108:2804–2810
Shao RG, Cao CX, Nieves-Neira W, Dimanche-Boitrel MT, Solary E, Pommier Y (2001) Activation of the Fas pathway independently of Fas ligand during apoptosis induced by camptothecin in p53 mutant human colon carcinoma cells. Oncogene 20:1852–1859
Hayakawa S, Saeki K, Sazuka M, Suzuki Y, Shoji Y, Ohta T et al (2001) Apoptosis induction by epigallocatechin gallate involves its binding to Fas. Biochem Biophys Res Commun 285:1102–1106
Coxon AB, Ward JM, Geradts J, Otterson GA, Zajac-Kaye M, Kaye FJ (1998) RET cooperates with RB/p53 inactivation in a somatic multi-step model for murine thyroid cancer. Oncogene 17:1625–1628
Zhang HY, Meng X, Du ZX, Fang CQ, Liu GL, Wang HQ et al (2009) Significance of survivin, caspase-3, and VEGF expression in thyroid carcinoma. Clin Exp Med 9:207–213
Rinner B, Siegl V, Pürstner P, Efferth T, Brem B, Greger H et al (2004) Activity of novel plant extracts against medullary thyroid carcinoma cells. Anticancer Res 24:495–500
Segouffin-Cariou C, Billaud M (2000) Transforming ability of MEN2A-RET requires activation of the phosphatidylinositol 3-kinase/AKT signaling pathway. J Biol Chem 275:3568–3576
Santoro M, Melillo RM, Carlomagno F, Fusco A, Vecchio G (2002) Molecular mechanisms of RET activation in human cancer. Ann N Y Acad Sci 963:116–121
Drosten M, Hilken G, Bokmann M, Rodicker F, Mise N, Cranston AN et al (2004) Role of MEN2A-derived RET in maintenance and proliferation of medullary thyroid carcinoma. J Natl Cancer Inst 96:1231–1239
Kunnimalaiyaan M, Ndiaye M, Chen H (2006) Apoptosis-mediated medullary thyroid cancer growth suppression by the PI3K inhibitor LY294002. Surgery 140:1009–1014
Furuya F, Lu C, Willingham MC, Cheng SY (2007) Inhibition of phosphatidylinositol 3-kinase delays tumor progression and blocks metastatic spread in a mouse model of thyroid cancer. Carcinogenesis 28:2451–2458
Liu Z, Hou P, Ji M, Guan H, Studeman K, Jensen K et al (2008) Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab 93:3106–3116
She M, Yang H, Sun L, Yeung SC (2006) Redox control of manumycin A-induced apoptosis in anaplastic thyroid cancer cells: involvement of the xenobiotic apoptotic pathway. Cancer Biol Ther 5:275–280
Schlumberger M, Carlomagno F, Baudin E, Bidart JM, Santoro M (2008) New therapeutic approaches to treat medullary thyroid carcinoma. Nat Clin Pract Endocrinol Metab 4:22–32
Adachi S, Nagao T, Ingolfsson HI, Maxfield FR, Andersen OS, Kopelovich L et al (2007) The inhibitory effect of (−)-epigallocatechin gallate on activation of the epidermal growth factor receptor is associated with altered lipid order in HT29 colon cancer cells. Cancer Res 67:6493–6501
Vitagliano D, De Falco V, Tamburrino A, Coluzzi S, Troncone G, Chiappetta G et al (2011) The tyrosine kinase inhibitor ZD6474 blocks proliferation of RET mutant medullary thyroid carcinoma cells. Endocr Relat Cancer 18(1):1–11
Bucci C, Thomsen P, Nicoziani P, McCarthy J, Deurs B (2000) Rab7: a key to lysosome biogenesis. Mol Biol Cell 11:467–480
Acknowledgments
Authors are thankful to Ms. R. Sarkar for editing the manuscript. Thanks are also due to Mr. R. Dutta, Mr. S. Samanta, Mrs. S. Das for technical help. This work was supported by research grants from Department of Biotechnology, Department of Science and Technology, Council of Scientific and Industrial Research, Government of India.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Minakshi Mazumdar and Arghya Adhikary contributed equally to this work.
Rights and permissions
About this article
Cite this article
Mazumdar, M., Adhikary, A., Chakraborty, S. et al. Targeting RET to induce medullary thyroid cancer cell apoptosis: an antagonistic interplay between PI3K/Akt and p38MAPK/caspase-8 pathways. Apoptosis 18, 589–604 (2013). https://doi.org/10.1007/s10495-013-0803-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-013-0803-0