Abstract
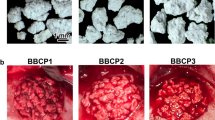
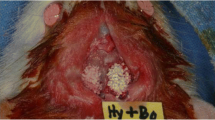
Our purpose was to evaluate the osteoconduction potential of mixed bovine bone (MBB) xenografts as an alternative for bone grafting of critical-size defects in the calvaria of rats. After surgery, in the time intervals of 1, 3, 6, and 9 months, rats were killed and their skulls collected, radiographed and histologically prepared for analysis. The data obtained from histological analysis reported that the particles of MBB did not promote an intense immunological response, evidencing its biocompatibility in rats. Our results clearly showed the interesting evidence that MBB was not completely reabsorbed at 9 months while a small amount of newly formed bone was deposited by osteoprogenitor cells bordering the defect. However, this discrete bone-forming stimulation was unable to regenerate the bone defect. Overall, our results suggest that the properties of MBB are not suitable for stimulating intense bone regeneration in critical bone defects in rats.





Similar content being viewed by others
References
Zambuzzi WF, Oliveira RC, Pereira FL, Cestari TM, Taga R, Granjeiro JM. Rat subcutaneous tissue response to macrogranular porous anorganic bovine bone graft. Braz Dent J. 2006;17(4):274–8.
Molly L, Vandromme H, Quirynen M, Schepers E, Adams JL, Van Steenberghe D. Bone formation following implantation of bone biomaterials into extraction sites. J Periodontol. 2008;79(6):1108–15.
Wenz B, Oesch B, Horst M. Analysis of the risk of transmitting bovine spongiform encephalopathy through bone grafts derived from bovine bone. Biomaterials. 2001;22(12):1599–606.
Anderson A, Mucalo MR, Johnson GS, Lorier MA. The processing and characterization of animal-derived bone to yield materials with biomedical applications. Part III: material and mechanical properties of fresh and processed bovine cancellous bone. J Mater Sci Mater Med. 2000;11(11):743–9.
Johnson GS, Mucalo MR, Lorier MA. The processing and characterization of animal-derived bone to yield materials with biomedical applications: part 1: modifiable porous implants from bovine condyle cancellous bone and characterization of bone materials as a function of processing. J Mater Sci Mater Med. 2000;11(7):427–41.
Meyer S, Floerkemeier T, Windhagen H. Histological osseointegration of Tutobone: first results in human. Arch Orthop Trauma Surg. 2008;128(6):539–44.
Le Geros RZ. Calcium phosphates in oral biology and medicine. Monographs in Oral Science. Karger. New York, USA, 1991.
Sciadini MF, Dawson JM, Johnson KD. Evaluation of bovine-derived bone protein with a natural coral carrier as a bone-graft substitute in a canine segmental defect model. J Orthop Res. 1997;15(6):844–57.
Jensen SS, Aaboe M, Pinholt EM, Hjorting-Hansen E, Melsen F, Ruyter IE. Tissue reactions and material characteristics of four bone substitutes. Int J Oral Maxillof Impl. 1996;11(1):55–66.
Cornelini R, Cangini F, Martuscelli G, Wennström J. Deproteinized bovine bone and biodegradable barrier membranes to support healing following immediate placement of transmucosal implants: a short-term controlled clinical trial. Int J Period Rest Dent. 2004;24(6):555–63.
Tadic D, Epple M. A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials. 2004;25(6):987–96.
de Oliveira RC, Carneiro E, Cestari TM, Taga R, Granjeiro JM. Dynamics of subcutaneous tissue response to the implantation of tetracycline-treated or untreated membrane of demineralized bovine cortical bone in rats. J Biomater Appl. 2006;21(2):167–78.
Luna LG. Manual of histologic staining methods of the armed forces institute of pathology. New York, USA: McGraw-Hill Book Company; 1968.
Frame JW. A convenient animal model for testing bone substitute materials. J Oral Surg. 1980;38(3):176–80.
Selvig KA. Discussion: animal models in reconstructive therapy. J Periodontol. 1994;65(12):1169–72.
Wang HL, Caroll WJ. Guided bone regeneration using bone grafts and collagen membranes. Quint Int. 2001;3(7):504–15.
Bosch C, Melsen B, Vargervik K. Importance of the critical-size bone defect in testing bone-regenerating materials. J Craniofac Surg. 1998;9(4):310–6.
Taga ML, Granjeiro JM, Cestari TM, Taga R. Healing of critical-size cranial defects in guinea pigs using a bovine bone-derived resorbable membrane. Int J Oral Maxillofac Impl. 2008;23(3):427–36.
Herculano MA, Tella OI Jr, Prandini MN, Alves MTS. Inhibition of peridural fibrosis after laminectomy using biological sheet in rat model. Arq Neuropsiquiatr. 2006;64(2A):259–63.
Zambuzzi WF, Paiva KB, Menezes R, Oliveira RC, Taga R, Granjeiro JM. MMP-9 and CD68(+) cells are required for tissue remodeling in response to natural hydroxyapatite. J Mol Histol. 2009;40(4):301–9.
Bertazzo S, Zambuzzi WF, Campos DD, Ferreira CV, Bertran CA. A simple method for enhancing cell adhesion to hydroxyapatite surface. Clin Oral Implants Res. 2010;21(12):1411–3.
Bertazzo S, Zambuzzi WF, Campos DD, Ogeda TL, Ferreira CV, Bertran CA. Hydroxyapatite surface solubility and effect on cell adhesion. Colloids Surf B Biointerfaces. 2010;78(2):177–84.
Bertazzo S, Zambuzzi WF, da Silva HA, Ferreira CV, Bertran CA. Bioactivation of alumina by surface modification: a possibility for improving the applicability of alumina in bone, oral repair. Clin Oral Implants Res. 2009;20(3):288–93.
Zambuzzi WF, Bruni-Cardoso A, Granjeiro JM, Peppelenbosch MP, de Carvalho HF, Aoyama H, Ferreira CV. On the road to understanding of the osteoblast adhesion: cytoskeleton organization is rearranged by distinct signaling pathways. J Cell Biochem. 2009;108(1):134–44.
Zambuzzi WF, Milani R, Teti A. Expanding the role of Src and protein-tyrosine phosphatases balance in modulating osteoblast metabolism: lessons from mice. Biochimie. 2010;92(4):327–32.
Milani R, Ferreira CV, Granjeiro JM, Paredes-Gamero EJ, Silva RA, Justo GZ, Nader HB, Galembeck E, Peppelenbosch MP, Aoyama H, Zambuzzi WF. Phosphoproteome reveals an atlas of protein signaling networks during osteoblast adhesion. J Cell Biochem. 2010;109(5):957–66.
Laquerriere P, Grandjean-Laquerriere A, Addadi-Rebbah S, Jallot E, Laurent-Maquin D, Frayssinet P, Guenounou M. MMP-2, MMP-9 and their inhibitors TIMP-2 and TIMP-1 production by human monocytes in vitro in the presence of different forms of hydroxyapatite particles. Biomaterials. 2004;25(13):2515–24.
Laquerriere P, Grandjean-Laquerriere A, Jallot E, Balossier G, Frayssinet P, Guenounou M. Importance of hydroxyapatite particles characteristics on cytokines production by human monocytes in vitro. Biomaterials. 2003;24(16):2739–47.
Nadra I, Boccaccini AR, Philippidis P, Whelan LC, McCarthy GM, Haskard DO, Landis RC. Effect of particle size on hydroxyapatite crystal-induced tumor necrosis factor alpha secretion by macrophages. Atherosclerosis. 2008;196(1):98–105.
Acknowledgments
The authors would like to thank Dr. Flávio Monteiro Amado and Miss Daniele Santi Ceolin for exceptional technical support. This work was supported by CAPES (CAPES Foundation), FAPESP (The State of São Paulo Research Foundation—99/10655-5, 01/10707-7, and 04/08095-1), CNPq (National Council for Scientific and Technological Development—350084/03-3, 475721/2003-9, 505350/04-1 and 504.808/04-4).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Accorsi-Mendonça, T., Zambuzzi, W.F., Bramante, C.M. et al. Biological monitoring of a xenomaterial for grafting: an evaluation in critical-size calvarial defects. J Mater Sci: Mater Med 22, 997–1004 (2011). https://doi.org/10.1007/s10856-011-4278-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-011-4278-7