Abstract
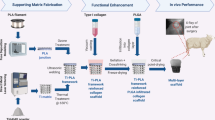
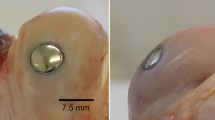
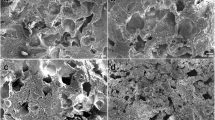
The purpose of this study was to evaluate the impact on osteochondral healing of press-fitted multiphasic osteochondral scaffolds consisting of poly(ester-urethane) (PUR) and hydroxyapatite into a cylindric osteochondral defect in the distal non-weight bearing femoral trochlear ridge of the rabbit. Two scaffolds were investigated, one with and one without an intermediate microporous membrane between the cartilage and the bone compartment of the scaffold. A control group without a scaffold placed into the defect was included. After 12 weeks macroscopic and histomorphological analyses were performed. The scaffold was easily press-fitted and provided a stable matrix for tissue repair. The membrane did not demonstrate a detrimental effect on tissue healing compared with the scaffold without membrane. However, the control group had statistically superior healing as reflected by histological differences in the cartilage and subchondral bone compartment between control group and each scaffold group. A more detailed analysis revealed that the difference was localized in the bone compartment healing. The present study demonstrates that an elastomeric PUR scaffold can easily be press-fitted into an osteochondral defect and provides a stable matrix for tissue repair. However, the multi-phasic scaffold did not provide a clear advantage for tissue healing. Future investigations should refine especially the bone phase of the implant to increase its stiffness, biocompatibility and osteoconductive activity. A more precise fabrication technique would be necessary for the matching of tissue organisation.







Similar content being viewed by others
References
Muir H. The chondrocyte, architect of cartilage. Biomechanics, structure, function and molecular biology of cartilage matrix macromolecules. Bioessays. 1995;17(12):1039–48.
Bonzani IC, George JH, Stevens MM. Novel materials for bone and cartilage regeneration. Curr Opin Chem Biol. 2006;10(6):568–75.
Chiang H, Jiang CC. Repair of articular cartilage defects: review and perspectives. J Formos Med Assoc. 2009;108(2):87–101.
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–95.
Dunlop DD, Manheim LM, Yelin EH, Song J, Chang RW. The costs of arthritis. Arthritis Rheum. 2003;49(1):101–13.
Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15–25.
Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthr Cartil. 2002;10(6):432–63.
Martin I, Miot S, Barbero A, Jakob M, Wendt D. Osteochondral tissue engineering. J Biomech. 2007;40(4):750–65.
Nooeaid P, Salih V, Beier JP, Boccaccini AR. Osteochondral tissue engineering: scaffolds, stem cells and applications. J Cell Mol Med. 2012;16(10):2247–70.
Kandel RA, Grynpas M, Pilliar R, Lee J, Wang J, Waldman S, et al. Repair of osteochondral defects with biphasic cartilage-calcium polyphosphate constructs in a sheep model. Biomaterials. 2006;27(22):4120–31.
Schaefer DJ, Klemt C, Zhang XH, Stark GB. Tissue engineering with mesenchymal stem cells for cartilage and bone regeneration. Chirurg. 2000;71(9):1001–8.
Boissard CI, Bourban PE, Tami AE, Alini M, Eglin D. Nanohydroxyapatite/poly(ester urethane) scaffold for bone tissue engineering. Acta Biomater. 2009;5(9):3316–27.
Eglin D, Grad S, Gogolewski S, Alini M. Farnesol-modified biodegradable polyurethanes for cartilage tissue engineering. J Biomed Mater Res A. 2010;92(1):393–408.
Grad S, Kupcsik L, Gorna K, Gogolewski S, Alini M. The use of biodegradable polyurethane scaffolds for cartilage tissue engineering: potential and limitations. Biomaterials. 2003;24(28):5163–71.
Hofmann A, Ritz U, Verrier S, Eglin D, Alini M, Fuchs S, et al. The effect of human osteoblasts on proliferation and neo-vessel formation of human umbilical vein endothelial cells in a long-term 3D co-culture on polyurethane scaffolds. Biomaterials. 2008;29(31):4217–26.
Lee CR, Grad S, Gorna K, Gogolewski S, Goessl A, Alini M. Fibrin-polyurethane composites for articular cartilage tissue engineering: a preliminary analysis. Tissue Eng. 2005;11(9–10):1562–73.
Laschke MW, Strohe A, Menger MD, Alini M, Eglin D. In vitro and in vivo evaluation of a novel nanosize hydroxyapatite particles/poly(ester-urethane) composite scaffold for bone tissue engineering. Acta Biomater. 2010;6(6):2020–7.
Laschke MW, Strohe A, Scheuer C, Eglin D, Verrier S, Alini M, et al. In vivo biocompatibility and vascularization of biodegradable porous polyurethane scaffolds for tissue engineering. Acta Biomater. 2009;5(6):1991–2001.
Deplaine H, Lebourg M, Ripalda P, Vidaurre A, Sanz-Ramos P, Mora G, et al. Biomimetic hydroxyapatite coating on pore walls improves osteointegration of poly(L-lactic acid) scaffolds. J Biomed Mater ResB Appl Biomater. 2013;101(1):173–86.
Hannink G, de Mulder EL, van Tienen TG, Buma P. Effect of load on the repair of osteochondral defects using a porous polymer scaffold. J Biomed Mater Res B Appl Biomater. 2012;100(8):2082–9.
Grad S, Loparic M, Peter R, Stolz M, Aebi U, Alini M. Sliding motion modulates stiffness and friction coefficient at the surface of tissue engineered cartilage. Osteoarthr Cartil. 2012;20(4):288–95.
Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;2:58–69.
van den Borne MP, Raijmakers NJ, Vanlauwe J, Victor J, de Jong SN, Bellemans J, et al. International Cartilage Repair Society (ICRS) and oswestry macroscopic cartilage evaluation scores validated for use in Autologous Chondrocyte Implantation (ACI) and microfracture. Osteoarthr Cartil. 2007;15(12):1397–402.
Mainil-Varlet P, Aigner T, Brittberg M, Bullough P, Hollander A, Hunziker E, et al. Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS). J Bone Joint Surg Am. 2003;2:45–57.
O’Driscoll SW, Keeley FW, Salter RB. Durability of regenerated articular cartilage produced by free autogenous periosteal grafts in major full-thickness defects in joint surfaces under the influence of continuous passive motion. A follow-up report at one year. J Bone Joint Surg Am. 1988;70(4):595–606.
Frenkel SR, Bradica G, Brekke JH, Goldman SM, Ieska K, Issack P, et al. Regeneration of articular cartilage–evaluation of osteochondral defect repair in the rabbit using multiphasic implants. Osteoarthr Cartil. 2005;13(9):798–807.
Krych AJ, Wanivenhaus F, Ng KW, Doty S, Warren RF, Maher SA. Matrix generation within a macroporous non-degradable implant for osteochondral defects is not enhanced with partial enzymatic digestion of the surrounding tissue: evaluation in an in vivo rabbit model. J Mater Sci Mater Med. 2013;24(10):2429–37.
Duan P, Pan Z, Cao L, He Y, Wang H, Qu Z, et al. The effects of pore size in bilayered poly(lactide-co-glycolide) scaffolds on restoring osteochondral defects in rabbits. J Biomed Mat Res Part A. 2013;101(12):3365–669.
Chang NJ, Lin CC, Li CF, Su K, Yeh ML. The effect of osteochondral regeneration using polymer constructs and continuous passive motion therapy in the lower weight-bearing zone of femoral trocheal groove in rabbits. Ann Biomed Eng. 2013;41(2):385–97.
Ikeda R, Fujioka H, Nagura I, Kokubu T, Toyokawa N, Inui A, et al. The effect of porosity and mechanical property of a synthetic polymer scaffold on repair of osteochondral defects. Int Orthop. 2009;33(3):821–8.
Ahern BJ, Parvizi J, Boston R, Schaer TP. Preclinical animal models in single site cartilage defect testing: a systematic review. Osteoarthr Cartil. 2009;17(6):705–13.
Chu CR, Szczodry M, Bruno S. Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev. 2010;16(1):105–15.
Rudert M. Histological evaluation of osteochondral defects: consideration of animal models with emphasis on the rabbit, experimental setup, follow-up and applied methods. Cells Tissues Organs. 2002;171(4):229–40.
Frisbie DD, Cross MW, McIlwraith CW. A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee. VetComp OrthopTraumatol. 2006;19(3):142–6.
Kock NB, Van Susante JL, Buma P, Van KA, Verdonschot N. Press-fit stability of an osteochondral autograft: influence of different plug length and perfect depth alignment. Acta Orthop. 2006;77(3):422–8.
Shao X, Goh JC, Hutmacher DW, Lee EH, Zigang G. Repair of large articular osteochondral defects using hybrid scaffolds and bone marrow-derived mesenchymal stem cells in a rabbit model. Tissue Eng. 2006;12(6):1539–51.
Zoetis T, Tassinari MS, Bagi C, Walthall K, Hurtt ME. Species comparison of postnatal bone growth and development. Birth Defects ResB Dev Reprod Toxicol. 2003;68(2):86–110.
Schlichting K, Schell H, Kleemann RU, Schill A, Weiler A, Duda GN, et al. Influence of scaffold stiffness on subchondral bone and subsequent cartilage regeneration in an ovine model of osteochondral defect healing. Am J Sports Med. 2008;36(12):2379–91.
Yang PJ, Temenoff JS. Engineering orthopedic tissue interfaces. Tissue Eng Part B Rev. 2009;15(2):127–41.
Acknowledgments
Markus Glarner for his help in the synthesis and preparation of PUR scaffolds. Dr. Giuseppino Fortunato from EMPA St-Gallen, Switzerland for the processing of the electrospun PUR membranes, Dr. Dirk Nehrbass and Nora Goudsouzian for the help with the histological processing and analysis of the samples. And the team of the Preclinical Facility of the AO-Research Institute for the support while performing the in vivo part of this study. The authors are supported by a consortium grant from the AO Exploratory Research Board.
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dresing, I., Zeiter, S., Auer, J. et al. Evaluation of a press-fit osteochondral poly(ester-urethane) scaffold in a rabbit defect model. J Mater Sci: Mater Med 25, 1691–1700 (2014). https://doi.org/10.1007/s10856-014-5192-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-014-5192-6