Abstract
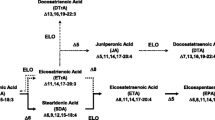
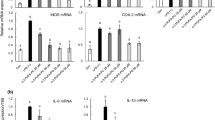
Eicosadienoic acid (Δ11,14–20:2; EDA) is a rare, naturally occurring n-6 polyunsaturated fatty acid (PUFA) found mainly in animal tissues. EDA is elongated from linoleic acid (LA), and can also be metabolized to dihomo-γ-linolenic acid (DGLA), arachidonic acid (AA), and sciadonic acid (Δ5,11,14–20:3; SCA). Although, the metabolism of EDA has been extensively studied, there are few reports regarding how EDA might affect inflammatory processes. The objective of this study was to determine the effect of EDA on the n-6 PUFA composition and inflammatory response of murine RAW264.7 macrophages to lipopolysaccharide (LPS). EDA was taken up rapidly by macrophages and metabolized to SCA, and the percentages of both fatty acids increased in cellular phospholipids in a dose-dependent manner. The incorporation of EDA into macrophage lipids increased the proportions of LA, DGLA, and AA as well, and reduced the proportion of total monounsaturated fatty acids. When LPS were applied to the macrophages, EDA decreased the production of nitric oxide (NO), and increased that of prostaglandin E2 (PGE2) and tumor necrotic factor-α. The modulation of NO and PGE2 was due, in part, to the modified expression of inducible nitric oxide synthase and type II cyclooxygenase. The differential effects of EDA on pro-inflammatory mediators might attribute to the negative feedback mechanism associated with prolonged inflammation. Furthermore, EDA was a weaker pro-inflammatory agent than LA, and not as anti-inflammatory as SCA. This study shows that EDA can modulate the metabolism of PUFA and alter the responsiveness of macrophages to inflammatory stimulation.







Similar content being viewed by others
References
Yagaloff KA, Franco L, Simko B, Burghardt B (1995) Essential fatty acids are antagonists of the leukotriene B4 receptor. Prostaglandins Leukot Essent Fat Acids 52:293–297
Brenner RR (1974) The oxidative desaturation of unsaturated fatty acids in animals. Mol Cell Biochem 3:41–52
Isseroff RR, Ziboh VA, Chapkin RS, Martinez DT (1987) Conversion of linoleic acid into arachidonic acid by cultured murine and human keratinocytes. J Lipid Res 28:1342–1349
Horrobin DF (1981) Loss of delta-6-desaturase activity as a key factor in aging. Med Hypotheses 7:1211–1220
Horrobin DF (1993) Fatty acid metabolism in health and disease: the role of delta-6-desaturase. Am J Clin Nutr 57:732S–736S
Chuang L-T, Leonard AE, Liu J-W, Mukerji P, Bray TM, Huang Y-S (2001) Inhibitory effect of conjugated linoleic acid on linoleic acid elongation in the transformed yeast with human elongase. Lipids 36:1099–1103
Kelder B, Mukerji P, Kirchner S, Hovanec G, Leonard AE, Chuang L-T, Kopchick JJ, Huang Y-S (2001) De novo synthesis of an essential fatty acid in cultured mammalian cells and transgenic animals. Mol Cell Biochem 219:7–11
Albert DH, Rhamy RK, Coniglio JG (1979) Desaturation of eicosa-11,14-dienoic acid in human testes. Lipids 14:498–500
Ullman D, Sprecher H (1971) An in vitro and in vivo study of the conversion of eicosa-11,14-dienoic acid to eicosa-5,11,14-trienoic acid and of the conversion of eicosa-11-enoic acid to eicosa-5,11-dienoic acid in the rat. Biochim Biophys Acta 248:186–197
Sprecher H, Lee C-J (1975) The absence of an 8-desaturases in rat liver: a reevaluation of optional pathways for the metabolism of linoleic and linolenic acids. Biochim Biophys Acta 388:113–125
Berger A, German JB (1991) Extensive incorporation of dietary delta-5,11,14 eicosatrienoate into the phosphatidylinositol pool. Biochim Biophys Acta 1085:371–376
Tanaka T, Takimoto T, Morishige J, Kikuta Y, Sugiura T, Satouchi K (1999) Non-methylene-interrupted polyunsaturated fatty acids: effective substitute for arachidonate of phosphatidylinositol. Biochem Biophys Res Commun 264:683–688
Tanaka T, Morishige J, Takimoto T, Takai Y, Satouchi K (2001) Metabolic characterization of sciadonic acid (5c,11c,14c-eicosatrienoic acid) as an effective substitute for arachidonate of phosphatidylinositol. Eur J Biochem 268:4928–4939
Berger A, Monnard I, Baur M, Charbonnet C, Safonova I, Jomard A (2002) Epidermal anti-inflammatory properties of 5,11,14 20:3: effect on mouse ear edema, PGE2 levels in cultured keratinocytes, and PPAR activation. Lipids Health Dis 1:5
Chuang L-T, Tsai P-J, Lee C-J, Huang Y-S (2009) Uptake and incorporation of pinolenic acid reduces n-6 polyunsaturated fatty acid and downstream prostaglandin formation in murine macrophage. Lipids 44:217–224
Folch J, Lees M, Sloane-Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitritie and [15N]nitrate in biological fluids. Anal Biochem 126:131–138
Chen Q, Yin FQ, Sprecher H (2000) The questionable role of a microsomal Δ8 acyl-CoA-dependent desaturase in the biosynthesis of polyunsaturated fatty acids. Lipids 35:871–879
Schenck PA, Rakoff H, Emken EA (1996) Delta 8 desaturation in vivo of deuterated eicosatrienoic acid by mouse liver. Lipids 31:593–600
Tanaka T, Morishige J, Iwawaki D, Fukuhara T, Hamamura N, Hirano K, Osumi T, Satouchi K (2007) Metabolic pathway that produces essential fatty acids from polymethylene-interrupted polyunsaturated fatty acids in animal cells. FEBS J 274:2728–2737
Chang C-S, Sun H-L, Lii C-K, Chen H-W, Chen P-Y, Liu K-L (2010) Gamma-linolenic acid inhibits inflammatory responses by regulating NF-κB and AP-1 activation in lipopolysaccharide-induced RAW 264.7 macrophages. Inflammation 33:46–57
Novak TE, Babcock TA, Jho DH, Helton WS, Espat NJ (2002) NF-κB inhibition by ω-3 fatty aids modulates LPS-stimulated macrophage TNF-α transcription. Am J Physiol Lung Cell Mol Physiol 284:L84–L89
Qi HY, Shelhamer JH (2005) Toll-like receptor 4 signaling regulates cytosolic phospholipase A2 activation and lipid generation in lipopolysaccharide-stimulated macrophages. J Biol Chem 280:38969–38975
Kitamura Y, Kakimura J, Matsuoka Y, Nomura Y, Gebicke-Haerter PJ, Taniguchi T (1999) Activators of peroxisome proliferator-activated receptor-gamma (PPARgamma) inhibit inducible nitric oxide synthase expression but increase heme oxygenase-1 expression in rat glial cells. Neurosci Lett 262:129–132
Shiraki T, Kamiya N, Shiki S, Kodama TS, Kakizuka A, Jingami H (2005) α,β-Unsaturated ketone is a core moiety of natural ligands for covalent binding to peroxisome proliferator-activated receptor gamma. J Biol Chem 280:14145–14153
Tetsuka T, Daphna-Iken D, Srivastava SK, Baier LD, Dumaine J, Morrison AR (1994) Cross-talk between cyclooxygenase and nitric oxide pathways: prostaglandin E2 negatively modulates induction of nitric oxide synthase by interleukin 1. Proc Natl Acad Sci USA 91:12168–12172
Milano S, Arcoleo F, Dieli M, D’Agostino R, D’Agostino P, De Nucci G, Cillari E (1995) Prostaglandin E2 regulates inducible nitric oxide synthase in the murine macrophage cell line J774. Prostaglandins 49:105–115
D’Acquisto F, Sautebin L, Iuvone T, Di Rosa M, Carnuccio R (1998) Prostaglandins prevent inducible nitric oxide synthase protein expression by inhibiting nuclear factor-κB activation in J774 macrophages. FEBS Lett 440:76–80
Petrie JR, Mackenzie AM, Shrestha P, Liu Q (2010) Isolation of three novel long-chain polyunsaturated fatty acid Δ9-elongases and the transgenic assembly of the entire Pavlova salina. Docosahexaenoic acid pathway in Nicotiana benthamiana. J Phycol 46:917–925
Acknowledgments
The authors thank Dr. Robert H. Glew for helpful comments and for editing the manuscript. The authors thank Ms. Shen-Yu Hsu for technical supports. This study was supported in part by research grants from the National Chung Hsing University, Taiwan, and the National Science Council, Taiwan, 98-1-4 and 99-2628-E-264-001, respectively.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, YS., Huang, WC., Li, CW. et al. Eicosadienoic acid differentially modulates production of pro-inflammatory modulators in murine macrophages. Mol Cell Biochem 358, 85–94 (2011). https://doi.org/10.1007/s11010-011-0924-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-0924-0