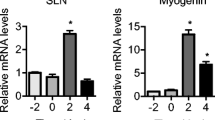
Abstract
Decorin inhibits the transforming growth factor superfamily to stimulate skeletal muscle hypertrophy. The endoplasmic reticulum (ER) plays a crucial role in the synthesis and folding of proteins. The disruption of ER homeostasis affects protein folding and causes ER stress, which is associated with protein synthesis in muscle cells. The purpose of this study was to establish the mechanism by which decorin promotes myoblast proliferation and myotube hypertrophy as mediated by an ER stress-related pathway. Duck myoblasts were incubated for 24 h with tunicamycin. Our results show that tunicamycin triggered ER stress in myoblasts and led to an increase in protein synthesis via a decorin-dependent pathway. We then transfected the decorin gene into duck myoblasts in vitro, and the results demonstrated that overexpression of duck decorin increased myogenic determination factor and follistatin expression and decreased myogenin and myostatin expression. Moreover, the results demonstrated that overexpression of duck decorin led to higher expression of ER stress marker genes. This increased expression trend can be alleviated in vitro by 4-PBA, which is a chemical chaperone that inhibits palmitate-induced ER stress. Collectively, our data show that duck decorin promotes myoblast proliferation mediated by an ER stress-related pathway.




Similar content being viewed by others
References
Lee SJ, Lee YS, Zimmers TA, Soleimani A, Matzuk MM, Tsuchida K, Cohn RD, Barton ER (2010) Regulation of muscle mass by follistatin and activins. Mol Endocrinol 24(10):1998–2008
Kollias HD, McDermott JC (2008) Transforming growth factor-β and myostatin signaling in skeletal muscle. J Appl Physiol 104(3):579–587
Yamaguchi Y, Mann DM, Ruoslahti E (1990) Negative regulation of transforming growth factor-β by the proteoglycan decorin. Nature 346(6281):281–284
Nicholas G, Thomas M, Langley B, Somers W, Patel K, Kemp CF, Sharma M, Kambadur R (2002) Titin-cap associates with, and regulates secretion of, Myostatin. J Cell Physiol 193(1):120–131
Amthor H, Nicholas G, McKinnell I, Kemp CF, Sharma M, Kambadur R, Patel K (2004) Follistatin complexes Myostatin and antagonises Myostatin-mediated inhibition of myogenesis. Dev Biol 270(1):19–30
Li Y, Li J, Zhu J, Sun B, Branca M, Tang Y, Foster W, Xiao X, Huard J (2007) Decorin gene transfer promotes muscle cell differentiation and muscle regeneration. Mol Ther 15(9):1616–1622
Deldicque L, Cani PD, Philp A, Raymackers JM, Meakin PJ, Ashford MLJ, Delzenne NM, Francaux M, Baar K (2010) The unfolded protein response is activated in skeletal muscle by high-fat feeding: potential role in the downregulation of protein synthesis. Am J Physiol Endocr Metab 299(5):E695–E705
Hendershot LM, Valentine VA, Lee AS, Morris SW, Shapiro DN (1994) Localization of the gene encoding human BiP/GRP78, the endoplasmic reticulum cognate of the HSP70 family, to chromosome 9q34. Genomics 20(2):281–284
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8(7):519–529
Nakanishi K, Sudo T, Morishima N (2005) Endoplasmic reticulum stress signaling transmitted by ATF6 mediates apoptosis during muscle development. Sci Signal 169(4):555–560
Eizirik DL, Cardozo AK, Cnop M (2008) The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 29(1):42–61
Deldicque L, Bertrand L, Patton A, Francaux M, Baar K (2011) ER stress induces anabolic resistance in muscle cells through PKB-induced blockade of mTORC1. PLoS ONE 6(6):e20993
Reed S, Senf S, Cornwell E, Kandarian S, Judge A (2011) Inhibition of IkappaB kinase alpha (IKKα) or IKKbeta (IKKβ) plus forkhead box O (Foxo) abolishes skeletal muscle atrophy. Biochem Biophys Res Commun 405(3):491–496
Novosyadlyy R, Kurshan N, Lann D, Vijayakumar A, Yakar S, LeRoith D (2008) Insulin-like growth factor-I protects cells from ER stress-induced apoptosis via enhancement of the adaptive capacity of endoplasmic reticulum. Cell Death Differ 15(8):1304–1317
Powers SK, Kavazis AN, McClung JM (2007) Oxidative stress and disuse muscle atrophy. J Appl Physiol 102(6):2389–2397
Rando TA, Blau HM (1994) Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol 125(6):1275–1287
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT. Method Methods 25(4):402–408
Scheuner D, Kaufman RJ (2008) The unfolded protein response: a pathway that links insulin demand with β-cell failure and diabetes. Endocr Rev 29(3):317–333
Sundar Rajan S, Srinivasan V, Balasubramanyam M, Tatu U (2007) Endoplasmic reticulum (ER) stress & diabetes. Indian J Med Res 125(3):411–424
Flamment M, Kammoun HL, Hainault I, Ferré P, Foufelle F (2010) Endoplasmic reticulum stress: a new actor in the development of hepatic steatosis. Curr Opin Lipidol 21(3):239–246
McPherron AC, Lee SJ (1997) Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci 94(23):12457–12461
Lee SJ (2007) Sprinting without myostatin: a genetic determinant of athletic prowess. Trends Genet 23(10):475–477
Gilson H, Schakman O, Kalista S, Lause P, Tsuchida K, Thissen JP (2009) Follistatin induces muscle hypertrophy through satellite cell proliferation and inhibition of both myostatin and activin. Am J Physiol-Endocr Metab 297(1):E157–E164
Lai E, Teodoro T, Volchuk A (2007) Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology 22(3):193–201
Harding HP, Ron D (2002) Endoplasmic reticulum stress and the development of diabetes a review. Diabetes 51(suppl 3):S455–S461
Watanabe Y, Suzuki O, Haruyama T, Akaike T (2003) Interferon-γ induces reactive oxygen species and endoplasmic reticulum stress at the hepatic apoptosis. J Cell Biochem 89(2):244–253
Ozcan U, Ozcan L, Yilmaz E, Düvel K, Sahin M, Manning BD, Hotamisligil GS (2008) Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell 29(5):541–551
Hage Hassan R, Hainault I, Vilquin JT, Samama C, Lasnier F, Ferré P, Foufelle F, Hajduch E (2011) Endoplasmic reticulum stress does not mediate palmitate-induced insulin resistance in mouse and human muscle cells. Diabetologia 1–11
Olguin HC, Santander C, Brandan E (2003) Inhibition of myoblast migration via decorin expression is critical for normal skeletal muscle differentiation. Dev Biol 259(2):209–224
Wyszyńska-Koko J, Pierzchała M, Flisikowski K, Kamyczek M, Różycki M, Kurył J (2006) Polymorphisms in coding and regulatory regions of the porcineMYF6 andMYOG genes and expression of theMYF6 gene inm. longissimus dorsi versus productive traits in pigs. J Appl Genet 47(2):131–138
Santra M, Reed CC, Iozzo RV (2002) Decorin binds to a narrow region of the epidermal growth factor (EGF) receptor, partially overlapping but distinct from the EGF-binding epitope. J Biol Chem 277(38):35671–35681
Lee SJ, McPherron AC (2001) Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA 98(16):9306–9311
Kishioka Y, Thomas M, Wakamatsu J, Hattori A, Sharma M, Kambadur R, Nishimura T (2008) Decorin enhances the proliferation and differentiation of myogenic cells through suppressing myostatin activity. J Cell Physiol 215(3):856–867
Lee SJ (2007) Quadrupling muscle mass in mice by targeting TGF-ß signaling pathways. PLoS ONE 2(8):e789
Gregor MF, Hotamisligil GS (2007) Thematic review series: Adipocyte Biology. Adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res 48(9):1905–1914
Appenzeller-Herzog C, Hall MN (2012) Bidirectional crosstalk between endoplasmic reticulum stress and mTOR signaling. Trends Cell Biol 22(5):274–282
Hotamisligil GS (2010) Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140(6):900–917
Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferré P, Foufelle F (2009) GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest 119(5):1201–1215
Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS (2006) Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313(5790):1137–1140
Acknowledgments
This work was supported by the National High Technology Research and Development Program of China (No. 2010AA10A109), the National Waterfowl Industrial Technology System (No. CARS-43-6), and the Program for Technology Innovative Research Team of Sichuan Province of China (2011JTD0032).
Author information
Authors and Affiliations
Corresponding author
Additional information
Lingli Sun and Kai Lu contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, L., Lu, K., Liu, H. et al. The effects of endoplasmic reticulum stress response on duck decorin stimulate myotube hypertrophy in myoblasts. Mol Cell Biochem 377, 151–161 (2013). https://doi.org/10.1007/s11010-013-1581-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-013-1581-2