Abstract
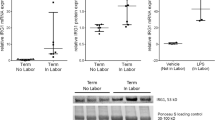
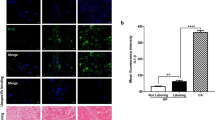
Term labour is associated with activation of inflammation which results in myometrial contractility, cervical ripening and decidual/membrane rupture. Serum amyloid A1 (SAA1) is an acute response protein, whose role and underlying regulatory mechanisms in human labour remain unknown. In this study, we found that the mRNA and protein expression of SAA1 in human myometrium at term was increased in labouring tissues compared to non-labouring tissues. In addition, the expression of SAA1 was significantly increased in human primary myometrial cells treated with the pro-inflammatory cytokines interleukin-1 beta (IL-1β) or tumour necrosis factor-alpha (TNF-α). Knockdown of SAA1 using siRNA (siSAA1) resulted in a significant reduction in the expression and secretion of pro-inflammatory cytokines (IL8, IL6), chemokines (CXCL5, CCL2), adhesion molecules (ICAM1, ICAM5) and contraction-associated factors (COX2, PGE2). Mechanistically, the effects of SAA1 were mediated through activation of the Yes-associated protein (YAP) pathway. There was a decrease in the protein expression of phosphorylated YAP (pYAP) after treatment of siSAA1-transfected human primary myometrial cells with IL-1β or TNF-α. Moreover, enhanced expression of YAP reversed the effect of siSAA1 on pro-labour mediators. In conclusion, these experiments demonstrated that SAA1 accelerates the inflammatory response associated with parturition by activating YAP pathway, which may be a novel understanding of the molecular mechanism of labour onset.








Similar content being viewed by others
References
Mathews TJ, MacDorman MF (2011) Infant mortality statistics from the 2007 period linked birth/infant death data set. Natl Vital Stat Rep 59(6):1–30
Blondel B, Lelong N, Kermarrec M, Goffinet F, National coordination Group of the National Perinatal Surveys (2012) Trends in perinatal health in France from 1995 to 2010. Results from the French National Perinatal Surveys. J Gynecol Obstet Biol Reprod (Paris) 41(4):e1–e15. https://doi.org/10.1016/j.jgyn.2012.04.014
Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, Mathers C, Black RE, Child Health Epidemiology Reference Group of WHO, Unicef (2012) Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 379(9832):2151–2161. https://doi.org/10.1016/S0140-6736(12)60560-1
Goldenberg RL, Culhane JF, Iams JD, Romero R (2008) Epidemiology and causes of preterm birth. Lancet 371(9606):75–84. https://doi.org/10.1016/S0140-6736(08)60074-4
Mwaniki MK, Atieno M, Lawn JE, Newton CR (2012) Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet 379(9814):445–452. https://doi.org/10.1016/S0140-6736(11)61577-8
Illanes SE, Perez-Sepulveda A, Rice GE, Mitchell MD (2014) Preterm labour association between labour physiology, tocolysis and prevention. Expert OpinInvestig Drugs 23(6):759–771. https://doi.org/10.1517/13543784.2014.905541
van Vliet EO, Boormans EM, de Lange TS, Mol BW, Oudijk MA (2014) Preterm labor: current pharmacotherapy options for tocolysis. Expert OpinPharmacother 15(6):787–797. https://doi.org/10.1517/14656566.2014.889684
Sivarajasingam SP, Imami N, Johnson MR (2016) Myometrial cytokines and their role in the onset of labour. J Endocrinol 231(3):R101–R119. https://doi.org/10.1530/JOE-16-0157
Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M (2006) The preterm parturition syndrome. BJOG 113(Suppl 3):17–42. https://doi.org/10.1111/j.1471-0528.2006.01120.x
Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, Norman JE (2003) Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod 9(1):41–45. https://doi.org/10.1093/molehr/gag001
Yuan M, Jordan F, McInnes IB, Harnett MM, Norman JE (2009) Leukocytes are primed in peripheral blood for activation during term and preterm labour. Mol Hum Reprod 15(11):713–724. https://doi.org/10.1093/molehr/gap054
Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, Norman JE (2002) Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod 66(2):445–449. https://doi.org/10.1095/biolreprod66.2.445
Flower L, Gray R, Pinkney J, Mohamed-Ali V (2003) Stimulation of interleukin-6 release by interleukin-1beta from isolated human adipocytes. Cytokine 21(1):32–37. https://doi.org/10.1016/s1043-4666(02)00495-7
Kniss DA, Zimmerman PD, Garver CL, Fertel RH (1997) Interleukin-1 receptor antagonist blocks interleukin-1-induced expression of cyclooxygenase-2 in endometrium. Am J Obstet Gynecol 177(3):559–567. https://doi.org/10.1016/s0002-9378(97)70146-7
Sun L, Ye RD (2016) Serum amyloid A1: structure, function and gene polymorphism. Gene 583(1):48–57. https://doi.org/10.1016/j.gene.2016.02.044
Urieli-Shoval S, Linke RP, Matzner Y (2000) Expression and function of serum amyloid A, a major acute-phase protein, in normal and disease states. CurrOpinHematol 7(1):64–69. https://doi.org/10.1097/00062752-200001000-00012
Hansen MT, Forst B, Cremers N, Quagliata L, Ambartsumian N, Grum-Schwensen B, Klingelhofer J, Abdul-Al A, Herrmann P, Osterland M, Stein U, Nielsen GH, Scherer PE, Lukanidin E, Sleeman JP, Grigorian M (2015) A link between inflammation and metastasis: serum amyloid A1 and A3 induce metastasis, and are targets of metastasis-inducing S100A4. Oncogene 34(4):424–435. https://doi.org/10.1038/onc.2013.568
Passey SL, Bozinovski S, Vlahos R, Anderson GP, Hansen MJ (2016) Serum amyloid A induces toll-like receptor 2-dependent inflammatory cytokine expression and atrophy in C2C12 skeletal muscle myotubes. PLoS ONE 11(1):e0146882. https://doi.org/10.1371/journal.pone.0146882
Yan Q, Sun L, Zhu Z, Wang L, Li S, Ye RD (2014) Jmjd3-mediated epigenetic regulation of inflammatory cytokine gene expression in serum amyloid A-stimulated macrophages. Cell Signal 26(9):1783–1791. https://doi.org/10.1016/j.cellsig.2014.03.025
De Buck M, Gouwy M, Wang JM, Van Snick J, Proost P, Struyf S, Van Damme J (2016) The cytokine-serum amyloid A-chemokine network. Cytokine Growth Factor Rev 30:55–69. https://doi.org/10.1016/j.cytogfr.2015.12.010
Shah C, Hari-Dass R, Raynes JG (2006) Serum amyloid A is an innate immune opsonin for Gram-negative bacteria. Blood 108(5):1751–1757. https://doi.org/10.1182/blood-2005-11-011932
Betts JC, Edbrooke MR, Thakker RV, Woo P (1991) The human acute-phase serum amyloid A gene family: structure, evolution and expression in hepatoma cells. Scand J Immunol 34(4):471–482. https://doi.org/10.1111/j.1365-3083.1991.tb01570.x
Sellar GC, Jordan SA, Bickmore WA, Fantes JA, van Heyningen V, Whitehead AS (1994) The human serum amyloid A protein (SAA) superfamily gene cluster: mapping to chromosome 11p15.1 by physical and genetic linkage analysis. Genomics 19(2):221–227. https://doi.org/10.1006/geno.1994.1051
Sack GH Jr (2018) Serum amyloid A—a review. Mol Med 24(1):46. https://doi.org/10.1186/s10020-018-0047-0
Gabay C, Kushner I (1999) Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340(6):448–454. https://doi.org/10.1056/NEJM199902113400607
Getz GS, Krishack PA, Reardon CA (2016) Serum amyloid A and atherosclerosis. CurrOpinLipidol 27(5):531–535. https://doi.org/10.1097/MOL.0000000000000331
Ye RD, Sun L (2015) Emerging functions of serum amyloid A in inflammation. J Leukoc Biol 98(6):923–929. https://doi.org/10.1189/jlb.3VMR0315-080R
Li W, Wang W, Zuo R, Liu C, Shu Q, Ying H, Sun K (2017) Induction of pro-inflammatory genes by serum amyloid A1 in human amnion fibroblasts. Sci Rep 7(1):693. https://doi.org/10.1038/s41598-017-00782-9
Wang YW, Wang WS, Wang LY, Bao YR, Lu JW, Lu Y, Zhang CY, Li WJ, Sun K, Ying H (2019) Extracellular matrix remodeling effects of serum amyloid A1 in the human amnion: Implications for fetal membrane rupture. Am J Reprod Immunol 81(1):e13073. https://doi.org/10.1111/aji.13073
Weiner CP, Mason CW, Dong Y, Buhimschi IA, Swaan PW, Buhimschi CS (2010) Human effector/initiator gene sets that regulate myometrial contractility during term and preterm labor. Am J Obstet Gynecol 202(5):474 e471–e420. https://doi.org/10.1016/j.ajog.2010.02.034
Tattersall M, Engineer N, Khanjani S, Sooranna SR, Roberts VH, Grigsby PL, Liang Z, Myatt L, Johnson MR (2008) Pro-labour myometrial gene expression: are preterm labour and term labour the same? Reproduction 135(4):569–579. https://doi.org/10.1530/REP-07-0461
Lim R, Lappas M (2019) Novel anti-inflammatory actions of TIPE2 in human primary amnion and myometrial cells. Reproduction 158(1):95–107. https://doi.org/10.1530/REP-19-0063
Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ (2006) Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol 195(6):1578–1589. https://doi.org/10.1016/j.ajog.2006.06.072
Lv Y, Kim K, Sheng Y, Cho J, Qian Z, Zhao YY, Hu G, Pan D, Malik AB, Hu G (2018) YAP controls endothelial activation and vascular inflammation through TRAF6. Circ Res 123(1):43–56. https://doi.org/10.1161/CIRCRESAHA.118.313143
Murakami S, Shahbazian D, Surana R, Zhang W, Chen H, Graham GT, White SM, Weiner LM, Yi C (2017) Yes-associated protein mediates immune reprogramming in pancreatic ductal adenocarcinoma. Oncogene 36(9):1232–1244. https://doi.org/10.1038/onc.2016.288
Taniguchi K, Wu LW, Grivennikov SI, de Jong PR, Lian I, Yu FX, Wang K, Ho SB, Boland BS, Chang JT, Sandborn WJ, Hardiman G, Raz E, Maehara Y, Yoshimura A, Zucman-Rossi J, Guan KL, Karin M (2015) A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature 519(7541):57–62. https://doi.org/10.1038/nature14228
Wang L, Luo JY, Li B, Tian XY, Chen LJ, Huang Y, Liu J, Deng D, Lau CW, Wan S, Ai D, Mak KK, Tong KK, Kwan KM, Wang N, Chiu JJ, Zhu Y, Huang Y (2016) Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature 540(7634):579–582. https://doi.org/10.1038/nature20602
Acknowledgements
This study was jointly funded by the National Natural Science Foundation of China (81873649) and Guangzhou Women's and Children's Medical Center fund innovation project (IP-2018-018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiang, Y., Pin, L., Shi, W. et al. SAA1 regulates pro-labour mediators in term labour by activating YAP pathway. Mol Cell Biochem 476, 2791–2801 (2021). https://doi.org/10.1007/s11010-021-04125-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-021-04125-1