Abstract
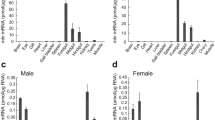
Cyclophilin A (CypA), a receptor for the immunosuppressive agent cyclosporin A (CsA), is a cis–trans peptidyl-prolyl isomerase (PPIase) which accelerates the cis–trans isomerization of prolyl-peptide bonds, interacts with a variety of proteins and therefore regulates their activities. One CypA (designated CfCypA) cDNA was cloned from Chlamys farreri by expressed sequence tag (EST) and rapid amplification of cDNA ends (RACE) techniques. The full-length cDNA of CfCypA consisted of 1,248 nucleotides with a canonical polyadenylation signal sequence AATAAA, a poly (A) tail, and an open reading frame (ORF) of 495 nucleotides encoding a polypeptide of 164 amino acids. The deduced amino acid sequence shared high similarity with CypA from the other species, indicating that CfCypA should be a new member of the CypA family. Quantitative real-time (RT) PCR was employed to assess the mRNA expression of CfCypA in various tissues and its temporal expression in haemocytes and gonad of scallops challenged with Vibrio anguillarum. The mRNA transcripts of CfCypA could be detected in all the examined tissues with highest expression level in gonad. After bacterial challenge, the expression level of CfCypA was almost unchanged in haemocytes, but up-regulated in gonad and increased to the peak (22.59-fold; P < 0.05) at 4 h post-injection, and then dropped to the original level at 8 h post-injection. These results indicated that CfCypA was constitutive expressed in haemocytes, but could be induced in gonad, and perhaps played a critical role in response to the bacterial challenge in gonad.




Similar content being viewed by others
References
Takahashi N, Hayano T, Suzuki M (1989) Peptidyl-prolyl cis–trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature 337:473–475. doi:10.1038/337473a0
Fischer G, Wittmann-Liebdd B, Lang K, Kiefhaber T, Schmid FX (1989) Cyclophilin and peptidyl-prolyl cis–trans isomerase are probably identical proteins. Nature 337:476–478. doi:10.1038/337476a0
Schonbrunner ER, Mayer S, Tropschug M, Fischer G, Takahashi N, Schmid FX (1991) Catalysis of protein folding by cyclophilins from different species. J Biol Chem 266:3630–3635
Steinmann B, Brucker P, Superti-Furga A (1991) Cyclosporin A slows collagen triple-helix formation in vivo: indirect evidence for a physiologic role of peptidyl-prolyl cis-trans isomerase. J Biol Chem 266:1299–1303
Schmid FX, Mayr LM, Mucke M, Schonbrunner ER (1993) Prolyl isomerases: role in protein folding. Adv Protein Chem 44:25–66. doi:10.1016/S0065-3233(08)60563-X
Price ER, Zydowsky LD, Jin MJ, Baker CH, McKeon FD, Walsh CT (1991) Human cyclophilin B: a second cyclophilin gene encodes a peptidyl-prolyl isomerase with a signal sequence. Proc Natl Acad Sci USA 88:1903–1907. doi:10.1073/pnas.88.5.1903
Uittenbogaard A, Ying YS, Smart EJ (1998) Characterization of a cytosolic heat-shock protein-caveolin chaperone complex. Involvement in cholesterol trafficking. J Biol Chem 273:6525–6532. doi:10.1074/jbc.273.11.6525
Montague JW, Hughes FM Jr, Cidlowski JA (1997) Native recombinant cyclophilins A, B, and C degrade DNA independently of peptidylprolyl cis–trans-isomerase activity. J Biol Chem 272:6677–6684. doi:10.1074/jbc.272.10.6677
Arora K, Gwinn WM, Bower MA, Watson A, Okwumabua I, MacDonald HR et al (2005) Extracellular cyclophilins contribute to the regulation of inflammatory responses. J Immunol 175:517–522
Flückiger S, Fijten H, Whitley P, Blaser K, Crameri R (2002) Cyclophilins, a new family of cross-reactive allergens. Eur J Immunol 32:10–17. doi:10.1002/1521-4141(200201)32:1<10::AID-IMMU10>3.0.CO;2-I
King TP, Hoffman D, Lowenstein H, Marsh DG, Platts-Mills TAE, Thomas W (1995) Allergen nomenclature. J Allergy Clin Immunol 95:5–14. doi:10.1016/S0091-6749(95)70027-7
Liu J, Walsh CT (1990) Peptidyl-prolyl cis–trans-isomerase from Escherichia coli: a periplasmic homolog of cyclophilin that is not inhibited by cyclosporin A. Proc Natl Acad Sci USA 87:4028–4032. doi:10.1073/pnas.87.11.4028
Hayano T, Takahashi N, Kato S, Maki N, Suzuki M (1991) Two distinct forms of peptidylprolyl-cis–trans-isomerase are expressed separately in periplasmic and cytoplasmic compartments of Escherichia coli cells. Biochemistry 30:3041–3048. doi:10.1021/bi00226a009
Haendler B, Keller R, Hiestand PC, Kocher HP, Wegmann G, Movva NR (1989) Yeast cyclophilin: isolation and characterization of the protein, cDNA and gene. Gene 83:39–46. doi:10.1016/0378-1119(89)90401-0
Tropschug M, Nicholson DW, Hartl FU, Köhler H, Pfanner N, Wachter E et al (1988) Cyclosporin A-binding protein (cyclophilin) of Neurospora crassa. J Biol Chem 263:14433–14440
Gasser CS, Gunning DA, Budelier KA, Brown SM (1990) Structure and expression of cytosolic cyclophilin/peptidyl-prolyl cis–trans isomerase of higher plants and production of active tomato cyclophilin in Escherichia coli. Proc Natl Acad Sci USA 87:9519–9523. doi:10.1073/pnas.87.24.9519
Chou IT, Gasser CS (1997) Characterization of the cyclophilin gene family of Arabidopsis thaliana and phylogenetic analysis of known cyclophilin proteins. Plant Mol Biol 35:873–892. doi:10.1023/A:1005930024796
Kong HY, Lee SC, Hwang BK (2001) Expression of pepper cyclophilin gene is differentially regulated during the pathogen infection and abiotic stress conditions. Physiol Mol Plant Pathol 59:189–199. doi:10.1006/pmpp.2001.0356
Johnson JC, Bhave M (2004) Characterization and physical mapping of cyclophilin A genes and identification of new classes of cyclophilins in wheat. J Cereal Sci 40:137–150. doi:10.1016/j.jcs.2004.05.002
Danielson PE, Forss-Petter S, Brow MA, Calavetta L, Douglass J, Milner RJ et al (1988) p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA 7:261–267
Shieh BH, Stamnes MA, Seavello S, Harris GL, Zuker CS (1989) The ninaA gene required for visual transduction in Drosophila encodes a homologue of cyclosporin A-binding protein. Nature 338:67–70. doi:10.1038/338067a0
Hasel KW, Sutcliffe JG (1990) Nucleotide sequence of a cDNA coding for mouse cyclophilin. Nucleic Acids Res 18:4019. doi:10.1093/nar/18.13.4019
Ryffel B, Woerly G, Greiner B, Haendler B, Mihatsch MJ, Foxwell BM (1991) Distribution of the cyclosporine binding protein cyclophilin in human tissues. Immunology 72:399–404
Kieffer LJ, Thalhammer T, Handschumacher RE (1992) Isolation and characterization of a 40-kDa cyclophilin-related protein. J Biol Chem 267:5503–5507
Yokoi H, Shimizu Y, Anazawa H, Lefebvre CA, Korneluk RG, Ikeda JE (1996) The structure and complete nucleotide sequence of the human Cyclophilin 40 (PPID) gene. Genomics 35:448–455. doi:10.1006/geno.1996.0384
Handschumacher RE, Harding MW, Rice J, Drugge RJ (1984) Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science 226:544–546. doi:10.1126/science.6238408
Sherry B, Yarlett N, Strupp A, Cerami A (1992) Identification of cyclophilin as a proinflammatory secretory product of lipopolysaccharide-activated macrophages. Proc Natl Acad Sci USA 89:3511–3515. doi:10.1073/pnas.89.8.3511
Liu J, Farmer JD, Lane WS Jr, Friedman J, Weissman I, Schreiber SL (1991) Calcineurin is a common target of cyclophilin–cyclosporin A and FKBP-FK506 complexes. Cell 66:807–815. doi:10.1016/0092-8674(91)90124-H
Jain J, Mecaffrey PG, Miner Z, Kerppola TK, Lamberts JN, Verdine GL et al (1993) The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature 365:352–355. doi:10.1038/365352a0
McCaffrey PG, Perrino BA, Soderling TR, Rao A (1993) NF-ATp, a T lymphocyte DNA-binding protein that is a target for calcineurin and immunosuppressive drugs. J Biol Chem 268:3747–3752
Colgan J, Asmal M, Neagu M, Yu B, Schneidkraut J, Lee Y et al (2004) Cyclophilin A regulates TCR signal strength in CD4+ T cells via a proline-directed conformational switch in Itk. Immunity 21:189–201. doi:10.1016/j.immuni.2004.07.005
Sokolskaja E, Sayah DM, Luban J (2004) Target cell cyclophilin A modulates human immunodeficiency virus type 1 infectivity. J Virol 78:12800–12808. doi:10.1128/JVI.78.23.12800-12808.2004
Gatanaga H, Das D, Suzuki Y, Yeh DD, Hussain KA, Ghosh AK et al (2006) Altered HIV-1 Gag protein interactions with cyclophilin A (CypA) on the acquisition of H219Q and H219P substitutions in the CypA binding loop. J Biol Chem 281:1241–1250. doi:10.1074/jbc.M505920200
Keckesova Z, Ylinen LMJ, Towers GJ (2006) Cyclophilin A renders human immunodeficiency virus type 1 sensitive to old world monkey but not human TRIM5α antiviral activity. J Virol 80:4683–4690. doi:10.1128/JVI.80.10.4683-4690.2006
Yeh HY, Klesius PH (2008) Channel Catfish, Ictalurus punctatus, cyclophilin A and B cDNA characterization and expression analysis. Vet Immunol Immunopathol 121:370–377. doi:10.1016/j.vetimm.2007.09.015
Zhang FS, Yang HS (1999) Strategic and counter measures to resolve mass mortality problems of Chlamys farreri. Mark Sci 2:38–42
Xiao J, Ford SE, Yang HS, Zhang GF, Zhang FS, Guo XM (2005) Studies on mass summer mortality of cultured zhikong scallops (Chlamys farreri Jones et Preston) in China. Aquaculture 250:602–615. doi:10.1016/j.aquaculture.2005.05.002
Fu CL, Song WB, Li Y, Zhang YZ, Yan HC (2004) Histopathological research and immunofluorescence of AVND virus infection in cultured scallop Chlamys farreri. Wei Sheng Wu Xue Bao 44:741–744
Yang XY, Li TB, Wang YL, Zhao ZY, Yang XS, Wang Y et al (1998) Preliminary study on the cause of scallop death. Shandong Fish 15:17–19
Zhang FS, Yang HS (1999) Analysis of the cause of mass mortality of farming Chlamys Farreri in summer in coastal areas of Shandong, China. Mark Sci 1:44–47
Ye JS, Wang XQ, Ma S, Yan BL (2007) Advances in research of epizootiology and measures of sustainable developmental breeding in bivalves. Anhui Agric Sci Bull 13:110–113
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24:4876–4882. doi:10.1093/nar/25.24.4876
Kumar S, Tamura K, Nei M (2004) MEGA3: Integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5:150–163. doi:10.1093/bib/5.2.150
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Felsenstein J (1985) Confidence limits on phylogenies: an approach using bootstrap. Evolution 39:783–791. doi:10.2307/2408678
Zhao JM, Song LS, Li CH, Ni DJ, Wu LT, Zhu L et al (2006) Molecular cloning, expression of a big defensin gene from bay scallop Argopecten irradians and the antimicrobial activity of its recombinant protein. Mol Immunol 44:360–368. doi:10.1016/j.molimm.2006.02.025
Eisenmesser EZ, Bosco DA, Akke M, Kern D (2002) Enzyme dynamics during catalysis. Science 295:1520–1523. doi:10.1126/science.1066176
Dornan J, Taylor P, Walkinshaw MD (2003) Structures of immunophilins and their ligand complexes. Curr Top Med Chem 3:1392–1409. doi:10.2174/1568026033451899
Tu HB, Yang WL, Jiang XY, Chen HP, Xiong Q, Wei JW et al (2003) Cloning, sequence analysis and evolutionary conservation of a full-length cDNA encoding cyclophilin A from red stingray Dasyatis akajei. Fish Shellfish Immunol 15:359–366. doi:10.1016/S1050-4648(02)00177-8
Piotukh K, Gu W, Kofler M, Labudde D, Helms V, Freund C (2005) Cyclophilin A binds to linear peptide motifs containing a consensus that is present in many human proteins. J Biol Chem 280:23668–23674. doi:10.1074/jbc.M503405200
Howard BR, Vajdos FF, Li S, Sundquist WI, Hill CP (2003) Structural insights into the catalytic mechanism of cyclophilin A. Nat Struct Biol 10:475–481. doi:10.1038/nsb927
Mark P, Nilsson L (2007) A molecular dynamics study of cyclophilin A free and in complex with the Ala-Pro dipeptide. Eur Biophys J 36:213–224. doi:10.1007/s00249-006-0121-3
Towers GJ (2007) The control of viral infection by tripartite motif proteins and cyclophilin A. Retrovirology 4:40–50. doi:10.1186/1742-4690-4-40
Jin ZG, Lungu AO, Xie L, Wang M, Wong C, Berk BC (2004) Cyclophilin A is a proinflammatory cytokine that activates endothelial cells. Arterioscler Thromb Vasc Biol 7:1186–1191. doi:10.1161/01.ATV.0000130664.51010.28
Martinez-Gonzalez J, Hegardt FG (1995) Characterization of a cDNA encoding a cytosolic peptidylprolyl cis–trans-isomerase from Blattella germanica. Eur J Biochem 234:284–292. doi:10.1111/j.1432-1033.1995.284_c.x
Qiu L, Jiang S, Huang J, Wang W, Zhu C, Su T (2008) Molecular cloning and mRNA expression of cyclophilin A gene in black tiger shrimp (Penaeus monodon). Fish Shellfish Immunol. doi:10.1016/j.fsi.2008.03.022
Tzou P, Ohresser S, Ferrandon D, Capovilla M, Reichhart JM, Lemaitre B et al (2000) Tissue specific inducible expression of antimicrobial peptide genes in Drosophila surface epithelia. Immunity 13:737–748. doi:10.1016/S1074-7613(00)00072-8
Lemaitre B, Hoffmann J (2007) The host defense of Drosophila melanogaster. Annu Rev Immunol 25:697–743. doi:10.1146/annurev.immunol.25.022106.141615
Acknowledgments
The authors would like to thank Dr. Jianguo Su and other laboratory members for technical advice and helpful discussion. This research was supported by 863 High Technology Project (No. 2006AA10A402) from the Chinese Ministry of Science and Technology, China, and grants (No. 30730070) from NSFC to Dr. Linsheng Song.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, X., Wang, L., Song, L. et al. A cyclophilin A inducible expressed in gonad of zhikong scallop Chlamys farreri . Mol Biol Rep 36, 1637–1645 (2009). https://doi.org/10.1007/s11033-008-9363-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-008-9363-8