Abstract
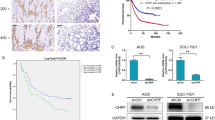
β-1,3-N-acetylglucosaminyltransferase-8(β3Gn-T8) catalyzes the transfer of GlcNAc to the non-reducing terminus of the Galβ1-4GlcNAc of tetraantennary N-glycan in vitro. It has been reported to be involved in malignant tumors, but a comprehensive understanding of how the glycolsyltransferase correlates with the invasive potential of human gastric cancer is not currently available. Therefore, we investigated the ability and possible mechanism involved with β3Gn-T8 in modulating matrix metalloproteinase-2 (MMP-2) and tissue inhibitor of metalloproteinase-2 (TIMP-2) in AGS gastric cancer cells. Here, we found out that siRNA-mediated suppression of the β3Gn-T8 could directly reduce the MMP-2 expression and activity as observed in RT-PCR, western blot and gelatin zymography analysis. Meanwhile, TIMP-2 expression had been increased. Cell invasion assay using matrigel matrix-coated transwell inserts showed that the invasive property was greatly suppressed in β3Gn-T8 siRNA transfected cells. Furthermore, cells overexpressing β3Gn-T8 gene (when transfected with pEGFP-C1 plasmid) also expressed MMP-2 gene, but TIMP-2 expression had been inhibited. The invasive ability of these cells was also enhanced. Protein–protein interaction analysis using STRING database showed that β3Gn-T8 and MMP-2 may have related signal pathway. In summary, our results reveal a new mechanism by which β3Gn-T8 can regulate MMP-2 and TIMP-2. We suggest that β3Gn-T8 can be used as a novel therapeutic target for human gastric treatment.






Similar content being viewed by others
References
Hakomori SI (1989) Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv Cancer Res 52:257–331
Hakomori SI (2002) Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci USA 99(16):10231–10233. doi:10.1073/pnas.172380699
Pierce M, Buckhaults P, Chen L, Fregien N (1997) Regulation of N-acetylglucosaminyltransferase V and Asn-linked oligosaccharide beta(l,6) branching by a growth factor signaling pathway and effects on cell adhesion and metastatic potential. Glycoconj J 14(5):623–630. doi:10.1023/A:1018592627696
Ishida H, Togayachi A, Sakai T, Iwai T, Hiruma T, Sato T, Okubo R, Inaba N, Kudo T, Gotoh M, Shoda J, anaka N, Narimatsu H (2005) A novel beta1,3-N-acetylglucosaminyltransferase (beta3Gn-T8), which synthesizes poly-N-acetyllactosamine, is dramatically upregulated in colon cancer. FEBS Lett 579:71–78. doi:10.1016/j.febslet.2004.11.037
Huang CQ, Zhou JL, Wu SL, Shan YX, Ten SL, Yu L (2004) Cloning and tissue distribution of the human B3GALT7 gene, a member of the β1,3-glycosyltransferase family. Glycoconj J 21:267–273. doi:10.1023/B:GLYC.0000045098.78968.4c
Hou R, Cao B, Chen Z, Li Y, Ning T, Li C, Xu C, Chen Z (2009) Association of cytotoxic T lymphocyte-associated antigen-4 gene haplotype with the susceptibility to gastric cancer. Mol Biol Rep 37(1):515–520. doi:10.1007/s11033-009-9705-1
Zhang L, Huang H, Wu K, Wang M, Wu B (2009) Impact of BTG2 expression on proliferation and invasion of gastric cancer cells in vitro. Mol Boil Rep. doi:10.1007/s11033-009-9777-y
Shi L, Zhao M, Luo Q, Ma YM, Zhong JL, Yuan XH, Huang CZ (2009) Overexpression of PIP5KL1 suppresses cell proliferation and migration in human gastric cancer cells. Mol Biol Rep. doi:10.1007/s11033-009-9701-5
Murray GI, Duncan ME, Arbuckle E, Melvin WT, Fothergill JE (1998) Matrix metalloproteinases and their inhibitors in gastric cancer. Gut 43:791–797. doi:10.1136/gut.43.6.791
Hart IR, Saini A (1992) Biology of tumour metastasis. Lancet 339:1453–1457. doi:10.1016/0140-6736(92)92039-I
Kohn EC, Liotta LA (1995) Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res 55:1856–1862
Li Y, Sun DL, Duan YN, Zhang XJ, Wang N, Zhou RM, Chen ZF, Wang SJ (2010) Association of functional polymorphisms in MMPs genes with gastric cardia adenocarcinoma and esophageal squamous cell carcinoma in high incidence region of North China. Mol Biol Rep 37(1):197–205. doi:10.1007/s11033-009-9593-4
Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S (1980) Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature 284:67–68. doi:10.1038/284067a0
Davies B, Miles DW, Happerfield LC, Naylor MS, Borrow LG, Rubens RD, Balkwill FR (1993) Activity of type IV collagenases in benign and malignant breast disease. Br J Cancer 67:1126–1131
Davies B, Waxman J, Wasan H, Abel P, Williams G, Krausz T, Neal D, Thomas D, Hanby A, Balkwill F (1993) Levels of matrix metalloproteinase in bladder cancer correlate with tumor grade and invasion. Cancer Res 53:5365–5369
Mrena J, Wiksten JP, Nordling S, Kokkola A, Ristimäki A, Haglund C (2006) MMP-2 but not MMP-9 associated with COX-2 and survival in gastric cancer. J Clin Pathol 59:618–623. doi:10.1136/jcp.2005.033761
Feng G, Tan Y (2000) Expression and significance of MMP2 and type IV collagen in gastric cancer. Zhonghua Wai Ke Za Zhi 38(10):775–777
Miao X, Yu C, Tan W, Xiong P, Liang G, Lu W, Lin D (2003) A functional polymorphism in the matrix metalloproteinase-2 gene promoter (−1306C/T) is associated with risk of development but not metastasis of gastric cardia adenocarcinoma. Cancer Res 63:3987–3990
Kubben FJGM, Sier CFM, Meijer MJW, van den Berg M, van der Reijden JJ, Griffioen G, van de Velde CJH, Lamers CBHW, Verspaget HW (2006) Clinical impact of MMP and TIMP gene polymorphisms in gastric cancer. Br J Cancer 95:744–751. doi:10.1038/sj.bjc.6603307
Douglas DA, Shi YE, Sang QA (1997) Computational sequence analysis of the tissue inhibitor of metallo-proteinase family. J Protein Chem 16(4):237–255. doi:10.1023/A:1026348808069
Morgunova E, Ari T, Ulrich B, Karl T (2002) Structural insight into the complex formation of latent matrix metalloproteinase 2 with tissue inhibitor of metalloproteinase 2. Proc Natl Acad Sci USA 99(11):7414–7419. doi:10.1073/pnas.102185399
Overall CM, Tam E, McQuibban GA, Morrison C, Wallon UM, Bigg HF, King AE, Roberts CR (2000) Domain interactions in the gelatinase A.TIMP-2.MT1-MMP activation complex. The ectodomain of the 44-kDa form of membrane type-1 matrix metalloproteinase does not modulate gelatinase A activation. J Biol Chem 275(50):39497–39506. doi:10.1074/jbc.M005932200
Bigg HF, Shi YE, Liu YE, Steffensen B, Overall CM (1997) Specific, high affinity binding of tissue inhibitor of metalloproteinases-4 (TIMP-4) to the COOH-terminal hemopexin-like domain of human gelatinase A. TIMP-4 binds progelatinase A and the COOH-terminal domain in a similar manner to TIMP-2. J Biol Chem 272(24):14500–15496. doi:10.1074/jbc.272.24.15496
Kai HS, Butler GS, Morrison CJ, King AE, Pelman GR, Overall CM (2002) Utilization of a novel recombinant myoglobin fusion protein expression system to characterize the tissue inhibitor of metalloproteinase (TIMP)-4 and TIMP-2 C-terminal domain and tails by mutagenesis. The importance of acidic residues in binding them. J Biol Chem 277(50):48696–48707. doi:10.1074/jbc.M209177200
Guo HB, Lee I, Kamar M, Akiyama SK, Pierce M (2002) Aberrant N-glycosylation of β1 integrin causes reduced α5β1 integrin clustering and stimulates cell migration. Cancer Res 62:6837–6845
Niu XD, Fan XD, Sun J, Ting P, Narula S, Lundell D (2004) Inhibition of fucosyltransferase VII by gallic acid and its derivatives. Arch Biochem Biophys 425(1):51–57. doi:10.1016/j.abb.2004.02.039
Albini A, Melchiori A, Santi L, Liotta LA, Brown PD, Stetler-Stevenson WG (1991) Tumor cell invasion inhibited by TIMP-2. J Natl Cancer Inst 83(11):775–779. doi:10.1093/jnci/83.11.775
Goñi J, Esteban FJ, de Mendizábal NV, Sepulcre J, Ardanza-Trevijano S, Agirrezabal I, Villoslada P (2008) A computational analysis of protein–protein interaction networks in neurodegenerative diseases. BMC Syst Biol 2(52):1752–1762. doi:10.1186/1752-0509-2-52
Dortay H, Mehnert N, Burkle L, Schmulling T, Heyl A (2006) Analysis of protein interactions within the cytokinin-signaling pathway of Arabidopsis thaliana. FEBS j 273(20):4631–4644. doi:10.1111/j.1742-4658.2006.05467.x
Kitano H (2007) A robustness-based approach to systems-oriented drug design. Nat Rev Drug Discov 6:202–210. doi:10.1038/nrd2195
von Mering C, Jensen LJ, Snel B, Hooper SD, Krupp M, Foglierini M, Jouffre N, Huynen MA, Bork P (2005) STRING known and predicted protein–protein associations, integrated and transferred across organisms. Nucleic Acids Res 33:433–437. doi:10.1093/nar/gki005
Young DF, Carlos TS, Hagmaier K, Fan L, Randall RE (2007) AGS and other tissue culture cells can unknowingly be persistently infected with PIV5; a virus that blocks interferon signalling by degrading STAT1. Virology 365(1):238–240. doi:10.1016/j.virol.2007.03.061
Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M (1994) A matrix metalloproteinase expressed on the surface of invasive tumor cells. Nature 370:61–65. doi:10.1038/370061a0
Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI (1995) Mechanism of cell surface activation of 72-kDa type IV collagenase isolation of the activated form of the membrane metalloprotease. J Biol Chem 270:5331–5338. doi:10.1074/jbc.270.10.5331
Goldberg GI, Marmer BL, Grant GA, Eisen AZ, Wilhelm S, He CS (1989) Human 72-KDa type IV collagenase forms a complex with a tissue inhibitor of metalloproteases designated TIMP-2. Proc Natl Acad Sci USA86(21):8207–8211
Trojanowska M (2000) Ets factors and regulation of the extracellular matrix. Oncogene 19(55):6464–6471
Behrens P, Rothe M, Wellmann A, Krischler J, Wernert N (2001) The Ets-1 transcription factor is up-regulated together with MMP 1 and MMP 9 in the stroma of pre-invasive breast cancer. J Pathol 194:43–50. doi:10.1002/path.844
Behrens P, Mathiak M, Mangold E, Kirdorf S, Wellmann A, Fogt F, Rothe M, Florin A, Wernert N (2003) Stromal expression of invasion-promoting, matrix-degrading proteases MMP-1 and -9 and the Ets 1 transcription factor in HNPCC carcinomas and sporadic colorectal cancers. Int J Cancer 107(2):183–188. doi:10.1002/ijc.11336
Okuducu AF, Zils U, Michaelis SA, Michaelides S, von Deimling A (2006) Ets-1 is up-regulated together with its target gene products matrix metalloproteinase-2 and matrix metalloproteinase-9 in atypical and anaplastic meningiomas. Histopathology 48(7):836–845. doi:10.1111/j.1365-2559.2006.02432.x
Zhang GS, Fahmy RG, diGirolamo N, Khachigian LM (2006) JUN siRNA regulates matrix metalloproteinase-2 expression, microvascular endothelial growth and retinal neovascularisation. J Cell Sci 119:3219–3226. doi:10.1242/jcs.03059
Zhang X, Zhang J, Yang X, Han X (2007) Several transcription factors regulate COX-2 gene expression in pancreatic b-cells. Mol Biol Rep 34:199–206. doi:10.1007/s11033-007-9085-3
Iwai T, Kudo T, Kawamoto R, Kubota T, Togayachi A, Hiruma T, Okada T, Kawamoto T, Morozumi K, Narimatsu H (2005) Core 3 synthase is down-regulated in colon carcinoma and profoundly suppresses the metastatic potential of carcinoma cells. Proc Natl Acad Sci USA102(12):4572–4577. doi:10.1073/pnas.0407983102
Ko JH, Miyoshi E, Noda K, Ekuni A, Kang RJ, Taniguchi N (1999) Regulation of the GnT-V promoter by transcription factor Ets-1 in various cancer cell lines. J Biol Chem 274:22941–22948. doi:10.1074/jbc.274.33.22941
Kang RJ, Saito H, Ihara Y, Miyoshi E, Koyama N, Sheng Y, Taniguchi N (1996) Transcriptional regulation of the N-acetylglucosaminyltransferase V gene in human bile duct carcinoma cells (HuCC-T1) is mediated by Ets-1. J Biol Chem 271:26706–26712. doi:10.1074/jbc.271.43.26706
Takeshi S, Kiyoshi F (2007) Sequential action of Ets-1 and Sp1 in the activation of the human β-1,4-galactosyltransferase V gene involved in abnormal glycosylation characteristic of cancer cells. J Biol Chem 282:27702–27712. doi:10.1074/jbc.M611862200
Seko A, Yamashita K (2008) Activation of β1,3-N-acetylglucosaminyltransferase-2 (β3Gn-T2) by β3Gn-T8. J Biol Chem 283:33094–33100. doi:10.1074/jbc.M806933200
Acknowledgment
This work was supported by grants from National Natural Science Foundation of China to Q.C. (No. 30670462).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, L., Liu, Z., Tu, Y. et al. Regulation of MMP-2 expression and activity by β-1,3-N-acetylglucosaminyltransferase-8 in AGS gastric cancer cells. Mol Biol Rep 38, 1541–1550 (2011). https://doi.org/10.1007/s11033-010-0262-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-010-0262-4