Abstract
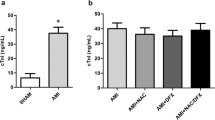
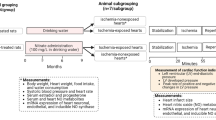
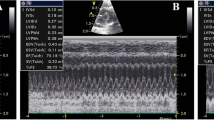
This study investigated the therapeutic potential of N-acetylcysteine (NAC) in the treatment of heart failure in female rats. Myocardial infarcted (MI) rats were given NAC (250 mg/kg/day p.o.) during 28 days after surgery (MI + NAC) or vehicle (MI + Placebo), and sham-operated rats received the same treatments (Sham + NAC and Sham + Placebo). Electrocardiographic and echocardiographic analyses were performed in the last week of treatment. Cardiac mRNA levels of types I and II superoxide dismutase (SOD), catalase, types I and III glutathione peroxidase (GPX), nerve growth factor (NGF), β1-adrenergic receptor (β1ADR), and type 2 muscarinic receptor (M2R) were assessed. Cardiac levels NADPH oxidase (NOX) activity, total content of reduced thiols, and SOD, GPX, and catalase activity were assessed. Compared to MI + Placebo group, MI + NAC group exhibited decreased NOX activity, increased content of reduced thiols, increased GPX activity, and normalized GPX III mRNA levels (p < 0.05). Heart and lung weights, left ventricular (LV) end-diastolic volume and left atrium/aorta ratio were decreased, while LV posterior wall thickness and ejection fraction were increased in MI + NAC group versus MI + Placebo rats (p < 0.05). Power density of low frequency band was decreased, while power density of high frequency and the root mean square of the successive differences were increased in MI + NAC rats versus MI + Placebo (p < 0.05). These findings indicate that NAC promotes therapeutic effects in the progression of MI-induced heart failure in female rats.




Similar content being viewed by others
References
Mendis S et al. (2011) Global Atlas on Cardiovascular disease prevention and control. World Health Organization PMID:21884856
Jneid H, Fonarow GC, Cannon CP, Hernandez AF, Palacios IF, Maree AO, Wells Q, Bozkurt B, LaBresh KA, Liang L, Hong Y, Newby LK, Fletcher G, Peterson E, Wexler L (2008) Sex differences in medical care and early death after acute myocardial infarction. Circulation 118:2803–2810. https://doi.org/10.1161/CIRCULATIONAHA.108.789800
Barretto ACP, Santos AC, Munhoz R, Rondon MUPB, Franco FG, Trombetta IC, Roveda F, de Matos LNJ, Braga AMW, Middlekauff HR, Negrão CE (2009) Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol 135:302–307. https://doi.org/10.1016/j.ijcard.2008.03.056
Levy D, Kannel KKL, Deedwania PC (1997) The progression from hypertension to heart failure. Am J Hypertens 10:280S-288S. https://doi.org/10.1001/jama.275.20.1557
Lund LH, Khush KK, Cherikh WS, Goldfarb S, Kucheryavaya AY, Levvey BJ, Meiser B, Rossano JW, Chambers DC, Yusen RD, Stehlik J (2017) The registry of the international society for heart and lung transplantation: thirty-fourth adult heart transplantation report—2017; focus theme: allograft ischemic time. J Hear Lung Transplant 36:1037–1046. https://doi.org/10.1016/j.healun.2017.07.019
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics Committee and Stroke Statistics Subcommittee, P (2017) Heart disease and stroke statistics-2017 update: a report from the american heart association. Circulation 135:e146–e603. https://doi.org/10.1161/CIR.0000000000000485
Junior RFR, Dabkowskia ER, Shekarb KC, O´Connella KA, Heckera PA, Murphyd MP (2018) MitoQ improves mitochondrial dysfunction in heart failure induced by pressure overload. Free Radic Biol Med 117(2018):18–29. https://doi.org/10.1016/j.freeradbiomed.2018.01.012
Qin F, Simeone M, Patel R (2007) Inhibition of NADPH oxidase reduces myocardial oxidative stress and apoptosis and improves cardiac function in heart failure after myocardial infarction. Free Radic Biol Med 43:271–281. https://doi.org/10.1016/j.freeradbiomed.2007.04.021
Hill MF, Singal PK (1996) Antioxidant and oxidative stress changes during heart failure subsequent to myocardial infarction in rats. Am J Pathol 148:291–300. https://doi.org/10.1161/01.CIR.96.7.2414
Li W, Kennedy D, Shao Z, Wang X, Kamdar AK, Weber M, Mislick K, Kiefer K, Morales R, Agatisa-Boyle B, Shih DM, Reddy ST, Moravec CS, Tang WHHW (2018) Paraoxonase 2 prevents the development of heart failure. Free Radic Biol Med 121:117–126. https://doi.org/10.1016/j.freeradbiomed.2018.04.583
Brookes PS, Digerness SB, Parks DA, Darley-Usmar V (2002) Mitochondrial function in response to cardiac ischemia-reperfusion after oral treatment with quercetin. Free Radic Biol Med 32:1220–1228. https://doi.org/10.1016/S0891-5849(02)00839-0
Fiorillo C, Becatti M, Pensalfini A, Cecchi C, Lanzilao L, Donzelli G, Nassi N, Giannini L, Borchi E, Nassi P (2008) Curcumin protects cardiac cells against ischemia-reperfusion injury: effects on oxidative stress, NF-κB, and JNK pathways. Free Radic Biol Med 45:839–846. https://doi.org/10.1074/jbc.M113.496570
Ardissino D, Merlini PA, Savonitto S, Demicheli G, Zanini P, Bertocchi F, Falcone C, Ghio S, Marinoni G, Montemartini C, Mussini A (1997) Effect of transdermal nitroglycerin or N-acetylcysteine, or both, in the long-term treatment of unstable angina pectoris. J Am Coll Cardiol 29:941–947. https://doi.org/10.1016/S0735-1097(97)00005-3
Horowitz JD, Henry CA, Syrjanen ML, Louis WJ, Fish D, Smith TW, Antman EM (1988) Combined use of nitroglycerin and N-acetylcysteine in the management of unstable angina pectoris. Circulation 77:787–794. https://doi.org/10.1161/01.CIR.77.4.787
Folts JD, Stamler J, Loscalzo J (1991) Intravenous nitroglycerin infusion inhibits cyclic blood flow responses caused by periodic platelet thrombus formation in stenosed canine coronary arteries. Circulation 83:2122–2127. https://doi.org/10.1161/01.CIR.83.6.2122
Brunet J, Boily MJÉ, Cordeau S, Des Rosiers C (1995) Effects of N-acetylcysteine in the rat heart reperfused after low-flow ischemia: evidence for a direct scavenging of hydroxyl radicals and a nitric oxide-dependent increase in coronary flow. Free Radic Biol Med 19:627–638. https://doi.org/10.1016/0891-5849(95)00077-B
Lee T-MTM, Lai P-YPY, Chang N-CNC (2010) Effect of N-acetylcysteine on sympathetic hyperinnervation in post-infarcted rat hearts. Cardiovasc Res 85:137–146. https://doi.org/10.1093/cvr/cvp286
Šochman J, Kolc J, Vrána M, Fabián J (1990) Cardioprotective effects of N-acetylcysteine: the reduction in the extent of infarction and occurrence of reperfusion arrhythmias in the dog. Int J Cardiol 28:191–196. https://doi.org/10.1016/0167-5273(90)90060-I
Giam B, Chu P.-Y, Kuruppu S, Smith A I, Horlock D, Kiriazis H, Du X.-J, Kaye DM, Rajapakse NW (2016) N‐ acetylcysteine attenuates the development of cardiac fibrosis and remodeling in a mouse model of heart failure. Physiol Rep 4:e12757. https://doi.org/https://doi.org/10.14814/phy2.12757
Becker LB, vanden Hoek TL, Shao ZH, Li CQ, Schumacker PT (1999) Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am J Physiol 277:H2240-6. https://doi.org/10.1152/ajpheart.1999.277.6.H2240
Griendling KK, Sorescu D, Ushio-Fukai M (2000) NAD (P) H oxidase: role in cardiovascular biology and disease. Circ Res 86:494–501 (PMID: 10720409)
Heymes C, Bendall JK, Ratajczak P, Cave AC, Samuel JL, Hasenfuss G, Shah AM (2003) Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol 41:2164–2171 (PMID: 12821241)
Benrahmoune M, Thérond P, Abedinzadeh Z (2000) The reaction of superoxide radical with N-acetylcysteine. Free Radic Biol Med 29:775–782. https://doi.org/10.1016/S0891-5849(00)00380-4
Aruoma OI, Halliwell B, Hoey BM, Butler J (1989) The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med 6:593–597. https://doi.org/10.1016/0891-5849(89)90066-X
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:1–2. https://doi.org/10.1016/0003-2697(76)90527-3
Atkuri KR, Mantovani JJ, Herzenberg LA, Herzenberg LA (2007) N-Acetylcysteine-a safe antidote for cysteine/glutathione deficiency. Curr Opin Pharmacol 7:355–359. https://doi.org/10.1016/j.coph.2007.04.005
Usal A, Acarturk E, Yuregir GT, Unlukurt I, Demirci C, Kurt HI, Birand A (1996) Decreased glutathione levels in acute myocardial infarction. Jpn Hear J 37:177–182. https://doi.org/10.1536/ihj.37.177
Okazaki T, Otani H, Shimazu T, Yoshioka K, Fujita M, Iwasaka T (2011) Ascorbic acid and N-acetyl cysteine prevent uncoupling of nitric oxide synthase and increase tolerance to ischemia/reperfusion injury in diabetic rat heart. Free Radic Res 45:1173–1183. https://doi.org/10.3109/10715762.2011.605361
Abe M, Takiguchi Y, Ichimaru S, Tsuchiya K, Wada K (2008) Comparison of the protective effect of N-acetylcysteine by different treatments on rat myocardial ischemia-reperfusion injury. J Pharmacol Sci 106:571–577. https://doi.org/10.1254/jphs.FP0071664
Kamunde C, Sharaf M, MacDonald N (2018) H2O2metabolism in liver and heart mitochondria: low emitting-high scavenging and high emitting-low scavenging systems. Free Radic Biol Med 124:135–148. https://doi.org/10.1016/j.freeradbiomed.2018.05.064
Lu J, Cheng K, Zhang B, Xu H, Cao Y, Guo F, Feng X, Xia Q (2015) Novel mechanisms for superoxide-scavenging activity of human manganese superoxide dismutase determined by the K68 key acetylation site. Free Radic Biol Med 85:114–126. https://doi.org/10.1016/j.freeradbiomed.2015.04.011
Giustarini D, Galvagni F, Dalle Donne I, Milzani A, Severi FM, Santucci A, Rossi R (2018) N-acetylcysteine ethyl ester as GSH enhancer in human primary endothelial cells: a comparative study with other drugs. Free Radic Biol Med 126:202–209. https://doi.org/10.1016/j.freeradbiomed.2018.08.013
Khaper N, Kaur K, Li T, Farahmand F, Singal PK (2003) Antioxidant enzyme gene expression in congestive heart failure following myocardial infarction. Mol Cell Biochem 251:9–15. https://doi.org/10.1023/A:1025448908694
Luo T, Liu H, Kim JK (2016) Estrogen protects the female heart from ischemia/reperfusion injury through manganese superoxide dismutase phosphorylation by mitochondrial p38β at threonine 79 and serine 106. PLoS One 11:e0167761. https://doi.org/10.1371/journal.pone.0167761
Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, Smieja M, Cambien F, Meyer J, Lackner KJ (2003) Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med 349:1605–1613. https://doi.org/10.1056/NEJMoa030535
Holley AS, Harding SA, Sasse A, Miller JH, Larsen PD (2016) Reduced glutathione peroxidase activity predicts increased cardiovascular risk following an acute coronary syndrome. Int Cardiovasc Forum J. https://doi.org/https://doi.org/10.17987/icfj.v6i0.241
Olivetti G, Capasso JM, Sonnenblick EH, Anversa P (1990) Side-to-side slippage of myocytes participates in ventricular wall remodeling acutely after myocardial infarction in rats. Circ Res 67:23–34 (PMID: 2364493)
Kono T, Sabbah HN, Rosman H, Alam M, Jafri S, Goldstein S (1992) Left ventricular shape is the primary determinant of functional mitral regurgitation in heart failure. J Am Coll Cardiol 20:1594–1598. https://doi.org/10.1016/0735-1097(92)90455-V
Miranda A, Costa-E-Sousa RH, Werneck-De-Castro JPS, Mattos EC, Olivares EL, Ribeiro VP, Silva MG, Goldenberg RCS, Campos-De-Carvalho AC (2007) Time course of echocardiographic and electrocardiographic parameters in myocardial infarct in rats. An Acad Bras Cienc 79:639–648. https://doi.org/10.1590/S0001-37652007000400006
Grossman W (1980) Cardiac hypertrophy: Useful adaptation or pathologic process? Am J Med 69:576–584. https://doi.org/10.1016/0002-9343(80)90471-4
Joachim H, Riede UN, Mittermayer C (1978) Das Lungengewicht A diagnostisches Kriterium (Abgrenzung der Schocklungen von Normallungen durch histologische, morphometrische and biochemische Untersuchungen). Pathol Res Pract 162:24–40. https://doi.org/10.1016/S0344-0338(78)80129-0
McKay RG, Pfeffer MA, Pasternak RC, Markis JE, Come PC, Nakao S, Alderman JD, Ferguson JJ, Safian RD, Grossman W (1986) Left ventricular remodeling after myocardial infarction: A corollary to infarct expansion. Circulation 74:693–702. https://doi.org/10.1161/01.CIR.74.4.693
Francis J, Weiss RM, Wei SG, Johnson AK, Felder RB (2001) Progression of heart failure after myocardial infarction in the rat. Am J Physiol Integr Comp Physiol 281:R1734–R1745. https://doi.org/10.1152/ajpregu.2001.281.5.R1734
Münzel T, Gori T, Keaney JF, Maack C, Daiber A (2015) Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur Heart J 36:2555–2564. https://doi.org/10.1093/eurheartj/ehv305
La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ (1998) Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet 351:478–484. https://doi.org/10.1016/S0140-6736(97)11144-8
Opthof T, Misier ARR, Coronel R, Vermeulen JT, Verberne HJ, Frank RGJ, Moulijn AC, Van Capelle FJL, Janse MJ (1991) Dispersion of refractoriness in canine ventricular myocardium: effects of sympathetic stimulation. Circ Res 68:1204–1215. https://doi.org/10.1161/01.RES.68.5.1204
Vaishnav S, Stevenson R, Marchant B, Lagi K, Ranjadayalan K, Timmis AD (1994) Relation between heart rate variability early after acute myocardial infarction and long-term mortality. Am J Cardiol 73:653–657. https://doi.org/10.1016/0002-9149(94)90928-8
Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH (1996) The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. carvedilol heart failure study group. N Engl J Med 334:1349–55. https://doi.org/10.1056/NEJM199605233342101
Zannad F, De Ferrari GM, Tuinenburg AE, Wright D, Brugada J, Butter C, Klein H, Stolen C, Meyer S, Stein KM, Ramuzat A, Schubert B, Daum D, Neuzil P, Botman C, Caste MA, D’Onofrio A, Solomon SD, Wold N, Ruble SB (2014) Chronic vagal stimulation for the treatment of low ejection fraction heart failure: results of the neural cardiac therapy for heart failure (NECTAR-HF) randomized controlled trial. Eur Heart J 36:425–433. https://doi.org/10.1093/eurheartj/ehu345
Levi-Montalcini R, Angeletti PU (1963) Essential role of the nerve growth factor in the survival and maintenance of dissociated sensory and sympathetic embryonic nerve cells in vitro. Dev Biol 7:653–659. https://doi.org/10.1016/0012-1606(63)90149-0
Zhou S, Chen LS, Miyauchi Y, Miyauchi M, Kar S, Kangavari S, Fishbein MC, Sharifi B, Chen PS (2004) Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res 95:76–83. https://doi.org/10.1161/01.RES.0000133678.22968.e3
Cao JM, Chen LS, KenKnight BH, Ohara T, Lee MH, Tsai J, Lai WW, Karagueuzian HS, Wolf PL, Fishbein MC, Chen PS (2000) Nerve sprouting and sudden cardiac death. Circ Res 86:816–821. https://doi.org/10.1161/01.RES.86.7.816
Colangelo AM, Johnson PF, Mocchetti I (1998) β-Adrenergic receptor-induced activation of nerve growth factor gene transcription in rat cerebral cortex involves CCAAT/enhancer-binding protein δ. Proc Natl Acad Sci 95:10920–10925. https://doi.org/10.1073/pnas.95.18.10920
Vinson C, Sigler P, McKnight S, Curran T (1989) Scissors-grip model for DNA recognition by a family of leucine zipper proteins. Science 246:911–916. https://doi.org/10.1126/science.2683088
Kamata H, Oka SI, Shibukawa Y, Kakuta J, Hirata H (2005) Redox regulation of nerve growth factor-induced neuronal differentiation of PC12 cells through modulation of the nerve growth factor receptor, TrkA. Arch Biochem Biophys 434:16–25. https://doi.org/10.1016/j.abb.2004.07.036
Olivares EL, Costa-e-Sousa RH, Werneck-de-Castro JPS, Pinho-Ribeiro V, Silva MG, Ribeiro KC, Mattos EC, Goldenberg RCS, Campos de Carvalho AC, Masuda MO (2007) Cellular cardiomyoplasty in large myocardial infarction: can the beneficial effect be enhanced by ACE-inhibitor therapy? Eur J Heart Fail 9:558–567. https://doi.org/10.1016/j.ejheart.2007.02.003
Souza N, Dos-Santos R, da Silveira ALB, Gantus MAV, Fortes F, Olivares EL (2016) Effects of autonomic balance and fluid and electrolyte changes on cardiac function in infarcted rats: a serial study of sexual dimorphism. Clin Exp Pharmacol Physiol 43:476–483. https://doi.org/10.1111/1440-1681.12543
Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-aho PO, Karjalainen PA (2014) Kubios HRV - Heart rate variability analysis software. Comput Methods Programs Biomed 113:210–220. https://doi.org/10.1016/j.cmpb.2013.07.024
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt J-U (2015) afbeelding 5. J Am Soc Echocardiogr 28:1-39.e14. https://doi.org/10.1016/j.echo.2014.10.003
Frankenfeld SSP, de Oliveira L, Ortenzi VH, Rego-Monteiro ICC, Chaves EEA, Ferreira AC, Leitão AC, Carvalho DP, Fortunato RSR (2014) The anabolic androgenic steroid nandrolone decanoate disrupts redox homeostasis in liver, heart and kidney of male wistar rats. PLoS One 9:e102699. https://doi.org/10.1371/journal.pone.0102699
Acknowledgements
This study was supported by the Department of Science and Technology - Brazilian Ministry of Health (DECIT/SCTIE/MS), the Brazilian Council for Scientific and Technological Development (CNPq), the Rio de Janeiro State Research Foundation (FAPERJ), the Coordination for the Improvement of Higher Education Personnel (CAPES).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11033_2020_5907_MOESM1_ESM.tif
Fig. S1 Antioxidant enzymes mRNA expression levels were restored by NAC therapy in infarcted hearts. mRNA expression levels of SOD1 (a), SOD2 (b), catalase (c), GPX1 (d), and GPX3 (e) were compared between Sham-operated (white bars) and MI rats (black bars) treated with placebo or NAC therapy (n = 5-7/group). Data are expressed as mean ± S.E.M. ***p < 0.001 versus Sham + Placebo group; #p < 0.05 versus MI + Placebo group (TIF 102 kb)
11033_2020_5907_MOESM2_ESM.tif
Fig. S2 NGF mRNA expression levels were attenuated by NAC therapy. mRNA expression levels of β1-adrenergic receptor (a), type 2 muscarinic receptor (b), and NGF (c) were compared between Sham-operated (white bars) and MI rats (black bars) treated with placebo or NAC therapy (n = 6/group). ***p < 0.001 versus Sham + Placebo group (TIF 95 kb)
Rights and permissions
About this article
Cite this article
Costa, C.R.M., Seara, F.A.C., Peixoto, M.S. et al. Progression of heart failure is attenuated by antioxidant therapy with N-acetylcysteine in myocardial infarcted female rats. Mol Biol Rep 47, 8645–8656 (2020). https://doi.org/10.1007/s11033-020-05907-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05907-4