Abstract
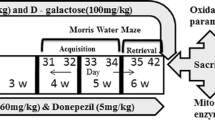
Oxidative stress initiates age-related reduction in hippocampal neurogenesis and the use of antioxidants has been proposed as an effective strategy to prevent or attenuate the reduction of neurogenesis in the hippocampus. In the present study, we investigated the effects of Cu,Zn-superoxide dismutase (SOD1) and/or peroxiredoxin-2 (PRX2) on cell proliferation and neuroblast differentiation in the dentate gyrus in a model of d-galactose-induced aging model. For this study, we constructed an expression vector, PEP-1, fused PEP-1 with SOD1 or PRX2, and generated PEP-1-SOD1 and PEP-1-PRX2 fusion protein. The aging model was induced by subcutaneous injection of d-galactose (100 mg/kg) to 6-week-old male mice for 10 weeks. PEP-1, PEP-1-SOD1 and/or PEP-1-PRX2 fusion protein was intraperitoneally administered to these mice at 13-week-old once a day for 3 weeks and sacrificed at 30 min after the last administrations. The administration of PEP-1-SOD1 and/or PEP-1-PRX2 significantly improved d-galactose-induced deficits on the escape latency, swimming speeds, platform crossings, spatial preference for the target quadrant in Morris water maze test. In addition, the administration of PEP-1-SOD1 and/or PEP-1-PRX2 ameliorated d-galactose-induced reductions of cell proliferation and neuroblast differentiation in the dentate gyrus and significantly reduced d-galactose-induced lipid peroxidation in the hippocampus. These effects were more prominent in the PEP-1-SOD1-treated group with PEP-1-PRX2. These results suggest that a SOD1 and/or PRX2 supplement to aged mice could improve the memory deficits, cell proliferation and neuroblast differentiation in the dentate gyrus of d-galactose induced aged mice by reducing lipid peroxidation.





Similar content being viewed by others
Abbreviations
- BDNF:
-
Brain-derived neurotrophic factor
- CREB:
-
cAMP response element-binding protein
- DCX:
-
Doublecortin
- d-gal:
-
d-galactose
- LTP:
-
Long-term potentiation
- MDA:
-
Malondialdehyde
- PCR:
-
Polymerase chain reaction
- PB:
-
Phosphate-buffer
- PBS:
-
Phosphate-buffered saline
- PRX:
-
Peroxiredoxin
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- SOD1:
-
Cu,Zn-superoxide dismutase
References
Altman J, Das GD (1965) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124:319–335
Eriksson PS, Perfilieva E, Björk-Eriksson T et al (1998) Neurogenesis in the adult human hippocampus. Nat Med 4:1313–1317
Bonfanti L, Peretto P (2011) Adult neurogenesis in mammals—a theme with many variations. Eur J Neurosci 34:930–950
Van Praag H, Schinder AF, Christie BR et al (2002) Functional neurogenesis in the adult hippocampus. Nature 415:1030–1034
Schmidt-Hieber C, Jonas P, Bischofberger J (2004) Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429:184–187
Kee N, Teixeira CM, Wang AH, Frankland PW (2007) Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci 10:355–362
Toni N, Teng EM, Bushong EA et al (2007) Synapse formation on neurons born in the adult hippocampus. Nat Neurosci 10:727–734
Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132:645–660
Kitamura T, Saitoh Y, Takashima N et al (2009) Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell 139:814–827
Halliwell B (1992) Reactive oxygen species and the central nervous system. J Neurochem 59:1609–1623
Butterfield DA, Sultana R (2007) Redox proteomics identification of oxidatively modified brain proteins in Alzheimer’s disease and mild cognitive impairment: insights into the progression of this dementing disorder. J Alzheimers Dis 12:61–72
Forster MJ, Dubey A, Dawson KM et al (1996) Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci USA 93:4765–4769
Knöferle J, Koch JC, Ostendorf T et al (2010) Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc Natl Acad Sci USA 107:6064–6069
Navarro A, Del Pino MJS, Gómez C, Peralta JL, Boveris A (2002) Behavioral dysfunction, brain oxidative stress, and impaired mitochondrial electron transfer in aging mice. Am J Physiol Regul Integr Comp Physiol 282:R985–R992
Nicolle MM, Gonzalez J, Sugaya K et al (2001) Signatures of hippocampal oxidative stress in aged spatial learning-impaired rodents. Neuroscience 107:415–431
Auerbach JM, Segal M (1997) Peroxide modulation of slow onset potentiation in rat hippocampus. J Neurosci 17:8695–8701
O’Donnell E, Vereker E, Lynch MA (2000) Age-related impairment in LTP is accompanied by enhanced activity of stress-activated protein kinases: analysis of underlying mechanisms. Eur J Neurosci 12:345–352
Watson JB, Khorasani H, Persson A et al (2002) Age-related deficits in long-term potentiation are insensitive to hydrogen peroxide: coincidence with enhanced autophosphorylation of Ca2+/calmodulin-dependent protein kinase II. J Neurosci Res 70:298–308
Watabe AM, O’Dell TJ (2003) Age-related changes in theta frequency stimulation-induced long-term potentiation. Neurobiol Aging 24:267–272
Kamsler A, Segal M (2003) Paradoxical actions of hydrogen peroxide on long-term potentiation in transgenic superoxide dismutase-1 mice. J Neurosci 23:10359–10367
Rosenzweig ES, Barnes CA (2003) Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol 69:143–179
Amrein I, Isler K, Lipp HP (2011) Comparing adult hippocampal neurogenesis in mammalian species and orders: influence of chronological age and life history stage. Eur J Neurosci 34:978–987
Drapeau E, Nora Abrous D (2008) Stem cell review series: role of neurogenesis in age-related memory disorders. Aging Cell 7:569–589
Smith J, Ladi E, Mayer-Proschel M, Noble M (2000) Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc Natl Acad Sci USA 97:10032–10037
Manda K, Ueno M, Anzai K (2009) Cranial irradiation-induced inhibition of neurogenesis in hippocampal dentate gyrus of adult mice: attenuation by melatonin pretreatment. J Pineal Res 46:71–78
Seaver LC, Imlay JA (2001) Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J Bacteriol 183:7173–7181
Botia B, Seyer D, Ravni A et al (2008) Peroxiredoxin 2 is involved in the neuroprotective effects of PACAP in cultured cerebellar granule neurons. J Mol Neurosci 36:61–72
Boulos S, Meloni BP, Arthur PG, Bojarski C, Knuckey NW (2007) Peroxiredoxin 2 overexpression protects cortical neuronal cultures from ischemic and oxidative injury but not glutamate excitotoxicity, whereas Cu/Zn superoxide dismutase 1 overexpression protects only against oxidative injury. J Neurosci Res 85:3089–3097
Fang J, Nakamura T, Cho DH, Gu Z, Lipton SA (2007) S-nitrosylation of peroxiredoxin 2 promotes oxidative stress-induced neuronal cell death in Parkinson’s disease. Proc Natl Acad Sci USA 104:18742–18747
Hattori F, Murayama N, Noshita T, Oikawa S (2003) Mitochondrial peroxiredoxin-3 protects hippocampal neurons from excitotoxic injury in vivo. J Neurochem 86:860–868
Kim SU, Jin MH, Kim YS et al (2011) Peroxiredoxin II preserves cognitive function against age-linked hippocampal oxidative damage. Neurobiol Aging 32:1054–1068
Joseph J, Cole G, Head E, Ingram D (2009) Nutrition, brain aging, and neurodegeneration. J Neurosci 29:12795–12801
Rutten BP, Steinbusch HW, Korr H, Schmitz C (2002) Antioxidants and Alzheimer’s disease: from bench to bedside (and back again). Curr Opin Clin Nutr Metab Care 5:645–651
Gahtan E, Auerbach JM, Groner Y, Segal M (1998) Reversible impairment of long-term potentiation in transgenic Cu/Zn-SOD mice. Eur J Neurosci 10:538–544
Levkovitz Y, Avignone E, Groner Y, Segal M (1999) Upregulation of GABA neurotransmission suppresses hippocampal excitability and prevents long-term potentiation in transgenic superoxide dismutase-overexpressing mice. J Neurosci 19:10977–10984
Yoo DY, Shin BN, Kim IH et al (2012) Effects of Cu,Zn-superoxide dismutase on cell proliferation and neuroblast differentiation in the mouse dentate gyrus. Neurochem Res 37:261–267
Song X, Bao M, Li D, Li YM (1999) Advanced glycation in D-galactose induced mouse aging model. Mech Ageing Dev 108:239–251
Bechmann I, Galea I, Perry VH (2007) What is the blood-brain barrier (not)? Trends Immunol 28:5–11
Eum WS, Kim DW, Hwang IK et al (2004) In vivo protein transduction: biologically active intact pep-1-superoxide dismutase fusion protein efficiently protects against ischemic insult. Free Radic Biol Med 37:1656–1669
Hwang IK, Eum WS, Yoo KY et al (2005) Copper chaperone for Cu,Zn-SOD supplement potentiates the Cu,Zn-SOD function of neuroprotective effects against ischemic neuronal damage in the gerbil hippocampus. Free Radic Biol Med 39:392–402
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Jeong HJ, Yoo DY, Kim DW et al. Neuroprotective effect of PEP-1-peroxiredoxin2 on CA1 regions in the hippocampus against ischemic insult. FEBS Lett, submitted
Brown JP, Couillard-Després S, Cooper-Kuhn CM et al (2003) Transient expression of doublecortin during adult neurogenesis. J Comp Neurol 467:1–10
Couillard-Despres S, Winner B, Schaubeck S et al (2005) Doulecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci 21:1–14
Yoo DY, Kim W, Lee CH et al (2012) Melatonin improves d-galactose-induced aging effects on behavior, neurogenesis, and lipid peroxidation in the mouse dentate gyrus via increasing pCREB expression. J Pineal Res 52:21–28
Franklin KBJ, Paxinos G (1997) The mouse brain in stereotaxic coordinates. Academic Press, San Diego
McCord JM, Fridovich I (1969) Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Knapp LT, Klann E (2002) Role of reactive oxygen species in hippocampal long-term potentiation: contributory or inhibitory? J Neurosci Res 70:1–7
Serrano F, Klann E (2004) Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res Rev 3:431–443
Halliwell B, Gutteridge JM (1990) Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol 186:1–85
Berlett BS, Stadtman ER (1997) Protein oxidation in aging, disease, and oxidative stress. J Biol Chem 272:20313–20316
Hamilton A, Holscher C (2012) The effect of ageing on neurogenesis and oxidative stress in the APPswe/PS1deltaE9 mouse model of Alzheimer’s disease. Brain Res 1449:83–93
Hsieh HM, Wu WM, Hu ML (2009) Soy isoflavones attenuate oxidative stress and improve parameters related to aging and Alzheimer’s disease in C57BL/6J mice treated with d-galactose. Food Chem Toxicol 47:625–632
Hu D, Klann E, Thiels E (2007) Superoxide dismutase and hippocampal function: age and isozyme matter. Antioxid Redox Signal 9:201–210
Knight JA (1998) Free radicals: their history and current status in aging and disease. Ann Clin Lab Sci 28:331–346
Cui X, Zuo P, Zhang Q et al (2006) Chronic systemic d-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: protective effects of R-alpha-lipoic acid. J Neurosci Res 84:647–654
Kamsler A, Avital A, Greenberger V, Segal M (2007) Aged SOD overexpressing mice exhibit enhanced spatial memory while lacking hippocampal neurogenesis. Antioxid Redox Signal 9:181–189
Jinno S (2011) Decline in adult neurogenesis during aging follows a topographic pattern in the mouse hippocampus. J Comp Neurol 519:451–466
Adachi M, Abe M, Sasaki T et al (2010) Role of inducible or neuronal nitric oxide synthase in neurogenesis of the dentate gyrus in aged mice. Metab Brain Dis 25:419–424
Walter J, Keiner S, Witte OW, Redecker C (2011) Age-related effects on hippocampal precursor cell subpopulations and neurogenesis. Neurobiol Aging 32:1906–1914
Eblin KE, Jensen TJ, Wnek SM et al (2009) Reactive oxygen species regulate properties of transformation in UROtsa cells exposed to monomethylarsonous acid by modulating MAPK signaling. Toxicology 255:107–114
Park IJ, Hwang JT, Kim YM, Ha J, Park OJ (2006) Differential modulation of AMPK signaling pathways by low or high levels of exogenous reactive oxygen species in colon cancer cells. Ann N Y Acad Sci 1091:102–109
Yoneyama M, Kawada K, Gotoh Y, Shiba T, Ogita K (2010) Endogenous reactive oxygen species are essential for proliferation of neural stem/progenitor cells. Neurochem Int 56:740–746
Contestabile A (2008) Regulation of transcription factors by nitric oxide in neurons and in neural-derived tumor cells. Prog Neurobiol 84:317–328
González A, Granados MP, Pariente JA, Salido GM (2006) H2O2 mobilizes Ca2+ from agonist- and thapsigargin-sensitive and insensitive intracellular stores and stimulates glutamate secretion in rat hippocampal astrocytes. Neurochem Res 31:741–750
Shetty PK, Huang FL, Huang KP (2008) Ischemia-elicited oxidative modulation of Ca2 +/calmodulin-dependent protein kinase II. J Biol Chem 283:5389–5401
Pellmar TC, Hollinden GE, Sarvey JM (1991) Free radicals accelerate the decay of long-term potentiation in field CA1 of guinea-pig hippocampus. Neuroscience 44:353–359
Rhee SG, Kang SW, Jeong W et al (2005) Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol 17:183–189
Fishman K, Baure J, Zou Y et al (2009) Radiation-induced reductions in neurogenesis are ameliorated in mice deficient in CuZnSOD or MnSOD. Free Radic Biol Med 47:1459–1467
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A2006995) and was supported by 2010 Research Grant from Kangwon National University.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jung Hoon Choi, Dae Won Kim and Dae Young Yoo have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Choi, J.H., Kim, D.W., Yoo, D.Y. et al. Repeated Administration of PEP-1-Cu,Zn-Superoxide Dismutase and PEP-1-Peroxiredoxin-2 to Senescent Mice Induced by d-galactose Improves the Hippocampal Functions. Neurochem Res 38, 2046–2055 (2013). https://doi.org/10.1007/s11064-013-1112-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-013-1112-2