Abstract
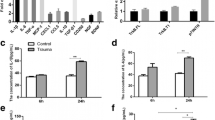
Brain edema and the associated increase in intracranial pressure are major consequences of traumatic brain injury (TBI) that accounts for most early deaths after TBI. We recently showed that acute severe trauma to cultured astrocytes results in cell swelling. We further examined whether trauma induces cell swelling in neurons and microglia. We found that severe trauma also caused cell swelling in cultured neurons, whereas no swelling was observed in microglia. While severe trauma caused cell swelling in both astrocytes and neurons, mild trauma to astrocytes, neurons, and microglia failed to cell swelling. Since extracellular levels of glutamate are increased in brain post-TBI and microglia are known to release cytokine, and direct exposure of astrocytes to these molecules are known to stimulate cell swelling, we examined whether glutamate or cytokines have any additive effect on trauma-induced cell swelling. Exposure of cultured astrocytes to trauma caused cell swelling, and such swelling was potentiated by the exposure of traumatized astrocytes to glutamate and cytokines. Conditioned medium (CM) from traumatized astrocytes had no effect on neuronal swelling post-trauma, while CM from traumatized neurons and microglia potentiated the effect of trauma on astrocyte swelling. Further, trauma significantly increased the Na–K–Cl co-transporter (NKCC) activity in neurons, and that inhibition of NKCC activity diminished the trauma-induced neuronal swelling. Our results indicate that a differential sensitivity to trauma-induced cell swelling exists in neural cells and that neurons and microglia are likely to be involved in the potentiation of the astrocyte swelling post-trauma.






Similar content being viewed by others
References
Marmarou A (2003) Pathophysiology of traumatic brain edema: current concepts. Acta Neurochir Suppl 86:7–10
Unterberg AW, Stroop R, Thomale UW, Kiening KL, Pauser S, Vollmann W (1997) Characterisation of brain edema following “controlled cortical impact injury” in rats. Acta Neurochir Suppl 70:106–108
Unterberg AW, Stover J, Kress B, Kiening KL (2004) Edema and brain trauma. Neuroscience 129(4):1021–1029
Barzo P, Marmarou A, Fatouros P, Hayasaki K, Corwin F (1997) Contribution of vasogenic and cellular edema to traumatic brain swelling measured by diffusion-weighted imaging. J Neurosurg 87(6):900–907
Jayakumar AR, Rao KV, Panickar KS, Moriyama M, Reddy PV, Norenberg MD (2008) Trauma-induced cell swelling in cultured astrocytes. J Neuropathol Exp Neurol 67(5):417–427
Jayakumar AR, Panickar KS, Curtis KM, Tong XY, Moriyama M, Norenberg MD (2011) Na-K-Cl cotransporter-1 in the mechanism of cell swelling in cultured astrocytes after fluid percussion injury. J Neurochem 117(3):437–448
Jayakumar AR, Tong XY, Ruiz-Cordero R, Bregy A, Bethea JR, Bramlett HM et al (2014) Activation of NF-kappaB mediates astrocyte swelling and brain edema in traumatic brain injury. J Neurotrauma 31(14):1249–1257
Panickar KS, Jayakumar AR, Norenberg MD (2002) Differential response of neural cells to trauma-induced free radical production in vitro. Neurochem Res 27(1–2):161–166
Gehrmann J, Banati RB, Wiessner C, Hossmann KA, Kreutzberg GW (1995) Reactive microglia in cerebral ischaemia: an early mediator of tissue damage? Neuropathol Appl Neurobiol 21(4):277–289
Hu S, Chao CC, Khanna KV, Gekker G, Peterson PK, Molitor TW (1996) Cytokine and free radical production by porcine microglia. Clin Immunol Immunopathol 78(1):93–96
Ducis I, Norenberg LO, Norenberg MD (1989) Effect of ammonium chloride on the astrocyte benzodiazepine receptor. Brain Res 493(2):362–365
Juurlink BH, Hertz L (1985) Plasticity of astrocytes in primary cultures: an experimental tool and a reason for methodological caution. Dev Neurosci 7(5–6):263–277
Schousboe AME, Drejer J, Hertz L (1989) Preparation of primary cultures of mouse (rat) cerebellar granule cells. In: Shahar A, DV J, Haber B, Vernadakis A, (eds) A dissection and tissue culture manual of the nervous system. Alan R. Liss, New York, pp 183–186
Flanary BE, Streit WJ (2004) Progressive telomere shortening occurs in cultured rat microglia, but not astrocytes. Glia 45(1):75–88
Sullivan HG, Martinez J, Becker DP, Miller JD, Griffith R, Wist AO (1976) Fluid-percussion model of mechanical brain injury in the cat. J Neurosurg 45(5):521–534
Shepard SR, Ghajar JB, Giannuzzi R, Kupferman S, Hariri RJ (1991) Fluid percussion barotrauma chamber: a new in vitro model for traumatic brain injury. J Surg Res 51(5):417–424
Jayakumar AR, Bak LK, Rama Rao KV, Waagepetersen HS, Schousboe A, Norenberg MD (2016) Neuronal cell death induced by mechanical percussion trauma in cultured neurons is not preceded by alterations in glucose, lactate and glutamine metabolism. Neurochem Res 41(1–2):307–315
Jayakumar AR, Tong XY, Shamaladevi N, Barcelona S, Gaidosh G, Agarwal A et al (2017) Defective synthesis and release of astrocytic thrombospondin-1 mediates the neuronal TDP-43 proteinopathy, resulting in defects in neuronal integrity associated with chronic traumatic encephalopathy: in vitro studies. J Neurochem 140(4):645–661
Kletzien RF, Pariza MW, Becker JE, Potter VR (1975) A method using 3-o-methyl-k-glucose and phloretin for the determination of intracellular water space of cells in monolayer culture. Anal Biochem 68(2):537–544
Kimelberg HK (1987) Anisotonic media and glutamate-induced ion transport and volume responses in primary astrocyte cultures. J Physiol 82(4):294–303
Norenberg MD, Baker L, Norenberg LO, Blicharska J, Bruce-Gregorios JH, Neary JT (1991) Ammonia-induced astrocyte swelling in primary culture. Neurochem Res 16(7):833–836
Sun D, Murali SG (1999) Na+-K+-2Cl- cotransporter in immature cortical neurons: a role in intracellular Cl- regulation. J Neurophysiol 81(4):1939–1948
Yi JH, Hazell AS (2006) Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem Int 48(5):394–403
Woodcock T, Morganti-Kossmann MC (2013) The role of markers of inflammation in traumatic brain injury. Front Neurol 4:18
Bachstetter AD, Rowe RK, Kaneko M, Goulding D, Lifshitz J, Van Eldik LJ (2013) The p38alpha MAPK regulates microglial responsiveness to diffuse traumatic brain injury. J Neurosci 33(14):6143–6153
Rama Rao KV, Jayakumar AR, Tong X, Alvarez VM, Norenberg MD (2010) Marked potentiation of cell swelling by cytokines in ammonia-sensitized cultured astrocytes. J Neuroinflamm 7:66
Jayakumar AR, Tong XY, Ospel J, Norenberg MD (2012) Role of cerebral endothelial cells in the astrocyte swelling and brain edema associated with acute hepatic encephalopathy. Neuroscience 218:305–316
Dorsett CR, McGuire JL, Niedzielko TL, DePasquale EA, Meller J, Floyd CL, McCullumsmith RE (2017) Traumatic brain injury induces alterations in cortical glutamate uptake without a reduction in glutamate transporter-1 protein expression. J Neurotrauma 34(1):220–234
Yi JH, Pow DV, Hazell AS (2005) Early loss of the glutamate transporter splice-variant GLT-1v in rat cerebral cortex following lateral fluid-percussion injury. Glia 49(1):121–133
Goodrich GS, Kabakov AY, Hameed MQ, Dhamne SC, Rosenberg PA, Rotenberg A (2013) Ceftriaxone treatment after traumatic brain injury restores expression of the glutamate transporter, GLT-1, reduces regional gliosis, and reduces post-traumatic seizures in the rat. J Neurotrauma 30(16):1434–1441
Rao VL, Başkaya MK, Doğan A, Rothstein JD, Dempsey RJ (1998) Traumatic brain injury down-regulates glial glutamate transporter (GLT-1 and GLAST) proteins in rat brain. J Neurochem 70(5):2020–2027
Karklin Fontana AC, Fox DP, Zoubroulis A, Valente Mortensen O, Raghupathi R (2016) Neuroprotective effects of the glutamate transporter activator (R)-(-)-5-methyl-1-nicotinoyl-2-pyrazoline (MS-153) following traumatic brain injury in the adult rat. J Neurotrauma 33(11):1073–1083
Maxwell WL, Bullock R, Landholt H, Fujisawa H (1994) Massive astrocytic swelling in response to extracellular glutamate—a possible mechanism for post-traumatic brain swelling? In: Ito U et al (eds) Brain edema IX. Acta Neurochirurgica, vol 60. Springer, Vienna
McKinney JS, Willoughby KA, Liang S, Ellis EF (1996) Stretch-induced injury of cultured neuronal, glial, and endothelial cells. Effect of polyethylene glycol-conjugated superoxide dismutase. Stroke 27:934–940
Zhang JR, Scherch HM, Hall ED (1996) Direct measurement of lipid hydroperoxides in iron-dependent spinal neuronal injury. J Neurochem 66:355–613
Arundine M, Aarts M, Lau A, Tymianski M (2004) Vulnerability of central neurons to secondary insults after in vitro mechanical stretch. J Neurosci 24:8106–8123
Jayakumar AR, Panickar KS, Murthy Ch R, Norenberg MD (2006) Oxidative stress and mitogen-activated protein kinase phosphorylation mediate ammonia-induced cell swelling and glutamate uptake inhibition in cultured astrocytes. J Neurosci 26(18):4774–4784
Hirrlinger J, Gutterer JM, Kussmaul L, Hamprecht B, Dringen R (2000) Microglial cells in culture express a prominent glutathione system for the defense against reactive oxygen species. Dev Neurosci 22(5–6):384–392
Acknowledgements
This work was supported by a Merit Review from the US Department of Veterans Affairs (MDN) and a Stanley J. Glaser grant (ARJ). The authors thank Alina Fernandez-Revuelta for the preparation of cell cultures and Xiaoying Tong for technical assistance.
Disclosure
KSP is currently employed at the Science & Technology Center, Hills PNC, a Colgate-Palmolive Company.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jayakumar, A.R., Taherian, M., Panickar, K.S. et al. Differential Response of Neural Cells to Trauma-Induced Swelling In Vitro. Neurochem Res 43, 397–406 (2018). https://doi.org/10.1007/s11064-017-2434-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2434-2