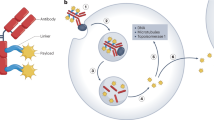
Abstract
Despite their side effect profile, there currently remains a heavy reliance on traditional cytotoxics and particularly tubulin targeting agents in cancer chemotherapy. To address this concern, significant progress has been made in the selective delivery of drugs to the tumour site. This review will examine the published data in support of the hypothesis that forming antibody conjugates of tubulin targeting agents is an effective approach towards their more effective delivery to the tumour site. Particular emphasis will be placed on the diversity of concepts under investigation, the efficacy of resultant conjugates, evidence of decreased resistance and the side effect profiles of the conjugates.










Similar content being viewed by others
Abbreviations
- ADC:
-
antibody-drug conjugate
- ALCL:
-
anaplastic large cell lymphoma
- BMPEO:
-
bis-maleimido-trioxyethylene glycol
- DM:
-
drug maytansinoid
- EGFR:
-
epidermal growth factor receptor
- HER2:
-
human epidermal growth factor receptor 2
- IgG:
-
immunoglobulin G
- mAb:
-
monoclonal antibody
- MC:
-
maleimidocaproyl
- MDR-1:
-
multidrug resistance protein-1
- MMAE:
-
monomethyl auristatin E
- MMAF:
-
monomethyl auristatin F
- MTD:
-
maximum tolerated dose
- PAB:
-
p-aminobenzyloxy carbonyl
- PABC:
-
p-aminobenzyl carbonyl
- PEG:
-
polyethylene glycol
- PSMA:
-
prostate specific membrane antigen
- Siglec:
-
sialic acid binding Ig-like lectins
- SMCC:
-
succinimidyl trans-4-(maleimidylmethyl) cyclohexane-1-carboxylate
- SPDB:
-
N-succinimidyl-3-(2-pyridylthio) propionate
- SPP:
-
N-succinimidyl 4-(2-pyridyldithio) pentanoate
- vc:
-
valine-citrulline
References
Chari RV. Targeted delivery of chemotherapeutics: tumor-activated prodrug therapy. Adv Drug Deliv Rev. 1998;31(1–2):89–104.
Kavallaris M. Microtubules and resistance to tubulin-binding agents. Nat Rev Cancer. 2010;10(3):194–204.
Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4(4):253–65.
Dumontet C, Jordan MA. Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discov. 2010;9(10):790–803.
Kavallaris M, Verrills NM, Hill BT. Anticancer therapy with novel tubulin-interacting drugs. Drug Resist Updat. 2001;4(6):392–401.
Lambert JM. Drug-conjugated monoclonal antibodies for the treatment of cancer. Curr Opin Pharmacol. 2005;5(5):543–9.
Vlahov IR, Wang Y, Kleindl PJ, Leamon CP. Design and regioselective synthesis of a new generation of targeted chemotherapeutics. Part II: Folic acid conjugates of tubulysins and their hydrazides. Bioorg Med Chem Lett. 2008;18(16):4558–61.
Kupchan SM, Komoda Y, Court WA, Thomas GJ, Smith RM, Karim A, et al. Tumor inhibitors. LXXIII. Maytansine, a novel antileukemic ansa macrolide from Maytenus ovatus. J Am Chem Soc. 1972;94(4):1354–6.
Oroudjev E, Lopus M, Wilson L, Audette C, Provenzano C, Erickson H, et al. Maytansinoid-antibody conjugates induce mitotic arrest by suppressing microtubule dynamic instability. Mol Cancer Ther. 2010;9(10):2700–13.
Lopus M. Antibody-DM1 conjugates as cancer therapeutics. Cancer Lett. 2011;307(2):113–8.
Chari RVJ, Martell BA, Gross JL, Cook SB, Shah SA, Blättler WA, et al. Immunoconjugates containing novel maytansinoids: promising anticancer drugs. Cancer Res. 1992;52(1):127–31.
Lambert JM. Antibody-maytansinoid conjugates as anticancer therapeutics: Proving their benefit. Hum Antibodies. 2007;16(1–2):5–8.
Yusuf RZ, Duan Z, Lamendola DE, Penson RT, Seiden MV. Paclitaxel resistance: molecular mechanisms and pharmacologic manipulation. Curr Cancer Drug Targets. 2003;3(1):1–19.
Argiris A, Duffy AG, Kummar S, Simone NL, Arai Y, Kim SW, et al. Early tumor progression associated with enhanced EGFR signaling with bortezomib, cetuximab, and radiotherapy for head and neck cancer. Clin Cancer Res. 2011;17(17):5755–64.
Kim KM, McDonagh CF, Westendorf L, Brown LL, Sussman D, Feist T, et al. Anti-CD30 diabody-drug conjugates with potent antitumor activity. Mol Cancer Ther. 2008;7(8):2486–97.
Guillemard V, Uri Saragovi H. Prodrug chemotherapeutics bypass p-glycoprotein resistance and kill tumors in vivo with high efficacy and target-dependent selectivity. Oncogene. 2004;23(20):3613–21.
Teicher BA, Chari RVJ. Antibody conjugate therapeutics: challenges and potential. Clin Cancer Res. 2011;17(20):6389–97.
Christiansen J, Rajasekaran AK. Biological impediments to monoclonal antibody-based cancer immunotherapy. Mol Cancer Ther. 2004;3(11):1493–501.
Francisco JA, Cerveny CG, Meyer DL, Mixan BJ, Klussman K, Chace DF, et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood. 2003;102(4):1458–65.
Ducry L, Stump B. Antibody−drug conjugates: linking cytotoxic payloads to monoclonal antibodies. Bioconjug Chem. 2009;21(1):5–13.
Webb S. Pharma interest surges in antibody drug conjugates. Nat Biotechnol. 2011;29(4):297–8.
Goldenberg DM. Monoclonal antibodies in cancer detection and therapy. Am J Med. 1993;94(3):297–312.
Wu X, Ojima I. Tumor specific novel taxoid-monoclonal antibody conjugates. Curr Med Chem. 2004;11(4):429–38.
Widdison WC, Wilhelm SD, Cavanagh EE, Whiteman KR, Leece BA, Kovtun Y, et al. Semisynthetic maytansine analogues for the targeted treatment of cancer. J Med Chem. 2006;49(14):4392–408.
Hamblett KJ, Senter PD, Chace DF, Sun MM, Lenox J, Cerveny CG, et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res. 2004;10(20):7063–70.
Tijink BM, Buter J, de Bree R, Giaccone G, Lang MS, Staab A, et al. A phase I dose escalation study with anti-CD44v6 bivatuzumab mertansine in patients with incurable squamous cell carcinoma of the head and neck or esophagus. Clin Cancer Res. 2006;12(20 Pt 1):6064–72.
Garanger E, Boturyn D, Dumy P. Tumor targeting with RGD peptide ligands-design of new molecular conjugates for imaging and therapy of cancers. Anticancer Agents Med Chem. 2007;7(5):552–8.
Yokota T, Milenic DE, Whitlow M, Schlom J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992;52(12):3402–8.
Adams GP, Schier R, Marshall K, Wolf EJ, McCall AM, Marks JD, et al. Increased affinity leads to improved selective tumor delivery of single-chain Fv antibodies. Cancer Res. 1998;58(3):485–90.
Junutula JR, Flagella KM, Graham RA, Parsons KL, Ha E, Raab H, et al. Engineered thio-trastuzumab-DM1 conjugate with an improved therapeutic index to target human epidermal growth factor receptor 2–positive breast cancer. Clin Cancer Res. 2010;16(19):4769–78.
Braun S, Schlimok G, Heumos I, Schaller G, Riethdorf L, Riethmuller G, et al. ErbB2 overexpression on occult metastatic cells in bone marrow predicts poor clinical outcome of stage I-III breast cancer patients. Cancer Res. 2001;61(5):1890–5.
Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, Weir S, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotech. 2008;26(8):925–32.
Schrama D, Reisfeld RA, Becker JC. Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov. 2006;5(2):147–59.
Erickson HK, Park PU, Widdison WC, Kovtun YV, Garrett LM, Hoffman K, et al. Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res. 2006;66(8):4426–33.
Lopus M, Oroudjev E, Wilson L, Wilhelm S, Widdison W, Chari R, et al. Maytansine and cellular metabolites of antibody-maytansinoid conjugates strongly suppress microtubule dynamics by binding to microtubules. Mol Cancer Ther. 2010;9(10):2689–99.
Kovtun YV, Audette CA, Ye Y, Xie H, Ruberti MF, Phinney SJ, et al. Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res. 2006;66(6):3214–21.
Kovtun YV, Goldmacher VS. Cell killing by antibody–drug conjugates. Cancer Lett. 2007;255(2):232–40.
Ikeda H, Hideshima T, Fulciniti M, Lutz RJ, Yasui H, Okawa Y, et al. The monoclonal antibody nBT062 conjugated to cytotoxic maytansinoids has selective cytotoxicity against CD138-positive multiple myeloma cells in vitro and in vivo. Clin Cancer Res. 2009;15(12):4028–37.
Lutz RJ, Whiteman KR. Antibody-maytansinoid conjugates for the treatment of myeloma. Mabs. 2009;1(6):548–51.
Beck A, Senter P, Chari R. World antibody drug conjugate Summit Europe: February 21-23, 2011; Frankfurt, Germany. Mabs. 2011;3(4):331–7.
Polson AG, Calemine-Fenaux J, Chan P, Chang W, Christensen E, Clark S, et al. Antibody-drug conjugates for the treatment of non-Hodgkin’s lymphoma: target and linker-drug selection. Cancer Res. 2009;69(6):2358–64.
Ma DS, Hopf CE, Malewicz AD, Donovan GP, Senter PD, Goeckeler WF, et al. Potent antitumor activity of an auristatin-conjugated, fully human monoclonal antibody to prostate-specific membrane antigen. Clin Cancer Res. 2006;12(8):2591–6.
Podgorski I, Sloane BF. Cathepsin B and its role(s) in cancer progression. Biochem Soc Symp. 2003;70:263–76.
Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, Chace DF, et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol. 2003;21(7):778.
Wahl AF, Klussman K, Thompson JD, Chen JH, Francisco LV, Risdon G, et al. The anti-CD30 monoclonal antibody SGN-30 promotes growth arrest and DNA fragmentation in vitro and affects antitumor activity in models of Hodgkin’s disease. Cancer Res. 2002;62(13):3736–42.
Doronina SO, Mendelsohn BA, Bovee TD, Cerveny CG, Alley SC, Meyer DL, et al. Enhanced activity of monomethylauristatin f through monoclonal antibody delivery: effects of linker technology on efficacy and toxicity. Bioconjug Chem. 2005;17(1):114–24.
Kovtun YV, Audette CA, Mayo MF, Jones GE, Doherty H, Maloney EK, et al. Antibody-maytansinoid conjugates designed to bypass multidrug resistance. Cancer Res. 2010;70(6):2528–37.
Zhao RY, Wilhelm SD, Audette C, Jones G, Leece BA, Lazar AC, et al. Synthesis and evaluation of hydrophilic linkers for antibody–maytansinoid conjugates. J Med Chem. 2011;54(10):3606–23.
Jeffrey SC, Andreyka JB, Bernhardt SX, Kissler KM, Kline T, Lenox JS, et al. Development and properties of β-glucuronide linkers for monoclonal antibody−drug conjugates. Bioconjug Chem. 2006;17(3):831–40.
Albin N, Massaad L, Toussaint C, Mathieu MC, Morizet J, Parise O, et al. Main drug-metabolizing enzyme systems in human breast tumors and peritumoral tissues. Cancer Res. 1993;53(15):3541–6.
Gilbert CW, McGowan EB, Seery GB, Black KS, Pegram MD. Targeted prodrug treatment of HER-2-positive breast tumor cells using trastuzumab and paclitaxel linked by A-Z-CINN™ Linker. J Exp Ther Oncol. 2003;3(1):27–35.
Ojima I, Geng X, Wu X, Qu C, Borella CP, Xie H, et al. Tumor-specific novel taxoid-monoclonal antibody conjugates. J Med Chem. 2002;45(26):5620–3.
Polson AG, Williams M, Gray AM, Fuji RN, Poon KA, McBride J, et al. Anti-CD22-MCC-DM1: an antibody-drug conjugate with a stable linker for the treatment of non-Hodgkin’s lymphoma. Leukemia. 2010;24(9):1566–73.
Law CL, Cerveny CG, Gordon KA, Klussman K, Mixan BJ, Chace DF, et al. Efficient elimination of B-lineage lymphomas by anti-CD20-auristatin conjugates. Clin Cancer Res. 2004;10(23):7842–51.
Yazawa N, Hamaguchi Y, Poe JC, Tedder TF. Immunotherapy using unconjugated CD19 monoclonal antibodies in animal models for B lymphocyte malignancies and autoimmune disease. Proc Natl Acad Sci USA. 2005;102(42):15178–83.
Gerber HP, Kung-Sutherland M, Stone I, Morris-Tilden C, Miyamoto J, McCormick R, et al. Potent antitumor activity of the anti-CD19 auristatin antibody drug conjugate hBU12-vcMMAE against rituximab-sensitive and -resistant lymphomas. Blood. 2009;113(18):4352–61.
Polson AG, Yu SF, Elkins K, Zheng B, Clark S, Ingle GS, et al. Antibody-drug conjugates targeted to CD79 for the treatment of non-Hodgkin lymphoma. Blood. 2007;110(2):616–23.
Dornan D, Bennett F, Chen Y, Dennis M, Eaton D, Elkins K, et al. Therapeutic potential of an anti-CD79b antibody-drug conjugate, anti-CD79b-vc-MMAE, for the treatment of non-Hodgkin lymphoma. Blood. 2009;114(13):2721–9.
Zheng B, Fuji RN, Elkins K, Yu S-F, Fuh FK, Chuh J, et al. In vivo effects of targeting CD79b with antibodies and antibody-drug conjugates. Mol Cancer Ther. 2009;8(10):2937–46.
Ab O, Bartle LM, Rui L, Coccia J, Johnson HA, Whiteman KR, et al. Abstract 4576: IMGN853, an anti-Folate Receptor I antibody-maytansinoid conjugate for targeted cancer therapy. Cancer Res. 2011;71(8 Supplement):4576.
Katz J, Janik JE, Younes A. Brentuximab vedotin (SGN-35). Clin Cancer Res. 2011;17(20):6428–36.
Liu C, Tadayoni BM, Bourret LA, Mattocks KM, Derr SM, Widdison WC, et al. Eradication of large colon tumor xenografts by targeted delivery of maytansinoids. Proc Natl Acad Sci USA. 1996;93(16):8618–23.
Tolcher AW, Ochoa L, Hammond LA, Patnaik A, Edwards T, Takimoto C, et al. Cantuzumab mertansine, a maytansinoid immunoconjugate directed to the CanAg Antigen: a Phase I, pharmacokinetic, and biologic correlative study. J Clin Oncol. 2003;21(2):211–22.
Robak T, Robak P, Smolewski P. TRU-016, a humanized anti-CD37 IgG fusion protein for the potential treatment of B-cell malignancies. Curr Opin Investig Drugs. 2009;10(12):1383–90.
Park PU, Yi Y, Li M, Chicklas S, Lai KC, Mayo MF, et al. Abstract 2830: antibody and linker selection for the anti-CD37 antibody-maytansinoid conjugate IMGN529 for the treatment of B-cell malignancies. Cancer Res. 2011;71(8 Supplement):2830.
Ireton RC, Chen J. EphA2 receptor tyrosine kinase as a promising target for cancer therapeutics. Curr Cancer Drug Targets. 2005;5(3):149–57.
Ogawa K, Pasqualini R, Lindberg RA, Kain R, Freeman AL, Pasquale EB. The ephrin-A1 ligand and its receptor, EphA2, are expressed during tumor neovascularization. Oncogene. 2000;19(52):6043.
Lee J-W, Stone RL, Lee SJ, Nam EJ, Roh J-W, Nick AM, et al. EphA2 targeted chemotherapy using an antibody drug conjugate in endometrial carcinoma. Clin Cancer Res. 2010;16(9):2562–70.
Junttila T, Li G, Parsons K, Phillips G, Sliwkowski M. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat. 2011;128(2):347–56.
Mathew J, Perez EA. Trastuzumab emtansine in human epidermal growth factor receptor 2-positive breast cancer: a review. Curr Opin Oncol. 2011;23(6):594–600.
Younes A, Yasothan U, Kirkpatrick P. Brentuximab vedotin. Nat Rev Drug Discov. 2012;11(1):19–20.
Oflazoglu E, Kissler KM, Sievers EL, Grewal IS, Gerber H-P. Combination of the anti-CD30-auristatin-E antibody-drug conjugate (SGN-35) with chemotherapy improves antitumour activity in Hodgkin lymphoma. Br J Haematol. 2008;142(1):69–73.
Blanc V, Bousseau A, Caron A, Carrez C, Lutz RJ, Lambert JM. SAR3419: an anti-CD19-Maytansinoid Immunoconjugate for the treatment of B-cell malignancies. Clin Cancer Res. 2011;17(20):6448–58.
Sanofi-Aventis. An Open Label Non-Randomized Phase 2 Study Evaluating SAR3419, an Anti-CD19 Antibody - Maytansine Conjugate, Administered as Single Agent by Intravenous Infusion to Patients With Relapsed or Refractory CD19+ Diffuse Large B-Cell Lymphoma. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 12 May 02]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01472887?term=SAR3419&rank=1 NLM Identifier: NCT01472887].
MedImmune LLC. Study of MEDI-547 to Evaluate the Safety, Tolerability, and Biologic Activity of IV Administration in Subjects With Relapsed or Refractory Solid Tumors. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000- [cited 12 Feb 23]. Available from: http://clinicaltrials.gov/ct2/show/NCT00796055?intr=%22MEDI-547%22&rank=1 NLM Identifier: NCT00796055.
Oflazoglu E, Stone IJ, Gordon K, Wood CG, Repasky EA, Grewal IS, et al. Potent anticarcinoma activity of the humanized anti-CD70 antibody h1F6 conjugated to the tubulin inhibitor Auristatin via an uncleavable linker. Clin Cancer Res. 2008;14(19):6171–80.
Ricart AD. Immunoconjugates against solid tumors: mind the gap. Clin Pharmacol Ther. 2011;89(4):513–23.
Acknowledgments and Disclosures
The authors would like to acknowledge funding from the Irish Council for Science Engineering and Technology (IRCSET) and Enterprise Ireland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stack, G.D., Walsh, J.J. Optimising the Delivery of Tubulin Targeting Agents through Antibody Conjugation. Pharm Res 29, 2972–2984 (2012). https://doi.org/10.1007/s11095-012-0810-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-012-0810-9