ABSTRACT
Purpose
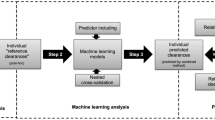
Recently, a covariate model characterizing developmental changes in clearance of amikacin in neonates has been developed using birth bodyweight and postnatal age. The aim of this study was to evaluate whether this covariate model can be used to predict maturation in clearance of other renally excreted drugs.
Methods
Five different neonatal datasets were available on netilmicin, vancomycin, tobramycin and gentamicin. The extensively validated covariate model for amikacin clearance was used to predict clearance of these drugs. In addition, independent reference models were developed based on a systematic covariate analysis.
Results
The descriptive and predictive properties of the models developed using the amikacin covariate model were good, and fairly similar to the independent reference models (goodness-of-fit plots, NPDE). Moreover, similar clearance values were obtained for both approaches. Finally, the same covariates as in the covariate model of amikacin, i.e. birth bodyweight and postnatal age, were identified on clearance in the independent reference models.
Conclusions
This study shows that pediatric covariate models may contain physiological information since information derived from one drug can be used to describe other drugs. This semi-physiological approach may be used to optimize sparse data analysis and to derive individualized dosing algorithms for drugs in children.






Similar content being viewed by others
Abbreviations
- bBW:
-
Birth bodyweight
- cBW:
-
Current bodyweight
- GFR:
-
Glomerular filtration rate
- NPDE:
-
Normalized prediction distribution error method
- PD:
-
Pharmacodynamics
- PK:
-
Pharmacokinetics
- PNA:
-
Postnatal age
REFERENCES
Cuzzolin L, Atzei A, Fanos V. Off-label and unlicensed prescribing for newborns and children in different settings: a review of the literature and a consideration about drug safety. Expert Opin Drug Saf. 2006;5(5):703–18.
‘t Jong GW, Vulto AG, de Hoog M, Schimmel KJ, Tibboel D, van den Anker JN. A survey of the use of off-label and unlicensed drugs in a Dutch children’s hospital. Pediatrics. 2001;108(5):1089–93.
Conroy S, Choonara I, Impicciatore P, Mohn A, Arnell H, Rane A, et al. Survey of unlicensed and off label drug use in paediatric wards in European countries. European Network for Drug Investigation in Children. BMJ. 2000;320(7227):79–82.
De Cock RF, Piana C, Krekels EH, Danhof M, Allegaert K, Knibbe CA. The role of population PK-PD modelling in paediatric clinical research. Eur J Clin Pharmacol. 2011;67 Suppl 1:5–16.
Ince I, de Wildt SN, Tibboel D, Danhof M, Knibbe CA. Tailor-made drug treatment for children: creation of an infrastructure for data-sharing and population PK-PD modeling. Drug Discov Today. 2009;14(5–6):316–20.
FDA C. Guidance for industry. General considerations for pediatric pharmacokinetic studies for drugs and biological products (Draft Guidance). 1998 [20 February 2013]; Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072114.pdf.
FDA C. Guidance for industry. Population pharmacokinetics. 1999; Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072137.pdf.
Tod M, Jullien V, Pons G. Facilitation of drug evaluation in children by population methods and modelling. Clin Pharmacokinet. 2008;47(4):231–43.
Smits A, Kulo A, de Hoon JN, Allegaert K. Pharmacokinetics of drugs in neonates: pattern recognition beyond compound specific observations. Curr Pharm Des. 2012;18(21):3119–46.
Manolis E, Osman TE, Herold R, Koenig F, Tomasi P, Vamvakas S, et al. Role of modeling and simulation in pediatric investigation plans. Paediatr Anaesth. 2011;21(3):214–21.
Bellanti F, Della Pasqua O. Modelling and simulation as research tools in paediatric drug development. Eur J Clin Pharmacol. 2011;67 Suppl 1:75–86.
Johnson TN. Modelling approaches to dose estimation in children. Br J Clin Pharmacol. 2005;59(6):663–9.
Knibbe CA, Krekels EH, Danhof M. Advances in paediatric pharmacokinetics. Expert Opin Drug Metab Toxicol. 2011;7(1):1–8.
Krekels EHJ, Neely M, Panoilia E, Tibboel D, Capparelli E, Danhof M, et al. From pediatric covariate model to semiphysiological function for maturation: part I—extrapolation of a covariate model from morphine to zidovudine. CPT: Pharmacometrics & Systems Pharmacology. 2012;1:e9. doi:10.1038/psp201211. Published online 3 October 2012.
Krekels EHJ, Johnson TN, den Hoedt SM, Rostami-Hodjegan A, Danhof M, Tibboel D, et al. From pediatric covariate model to semiphysiological function for maturation: part II—sensitivity to physiological and physicochemical properties. CPT: Pharmacometrics & Systems Pharmacology. 2012;1:e10. doi:10.1038/psp201212. Published online 10 October 2012.
De Cock RF, Allegaert K, Schreuder MF, Sherwin CM, de Hoog M, van den Anker JN, et al. Maturation of the glomerular filtration rate in neonates, as reflected by amikacin clearance. Clin Pharmacokinet. 2012;51(2):105–17.
Sherwin CM, Broadbent RS, Medlicott NJ, Reith DM. Individualising netilmicin dosing in neonates. Eur J Clin Pharmacol. 2008;64(12):1201–8.
de Hoog M, Schoemaker RC, Mouton JW, van den Anker JN. Tobramycin population pharmacokinetics in neonates. Clin Pharmacol Ther. 1997;62(4):392–9.
Allegaert K, Anderson BJ, van den Anker JN, Vanhaesebrouck S, de Zegher F. Renal drug clearance in preterm neonates: relation to prenatal growth. Ther Drug Monit. 2007;29(3):284–91.
Sherwin CM, McCaffrey F, Broadbent RS, Reith DM, Medlicott NJ. Discrepancies between predicted and observed rates of intravenous gentamicin delivery for neonates. J Pharm Pharmacol. 2009;61(4):465–71.
Nielsen EI, Sandstrom M, Honore PH, Ewald U, Friberg LE. Developmental pharmacokinetics of gentamicin in preterm and term neonates: population modelling of a prospective study. Clin Pharmacokinet. 2009;48(4):253–63.
Krekels EH, van Hasselt JG, Tibboel D, Danhof M, Knibbe CA. Systematic evaluation of the descriptive and predictive performance of paediatric morphine population models. Pharm Res. 2011;28(4):797–811.
Montgomery DC, Peck EA. Introduction to linear regression analysis. New York: Wiley; 1982. p. 301–2.
Karlsson MO, Savic RM. Diagnosing model diagnostics. Clin Pharmacol Ther. 2007;82(1):17–20.
Allegaert K. The impact of ibuprofen or indomethacin on renal drug clearance in neonates. J Matern Fetal Neonatal Med. 2009;22 Suppl 3:88–91.
Capparelli EV, Lane JR, Romanowski GL, McFeely EJ, Murray W, Sousa P, et al. The influences of renal function and maturation on vancomycin elimination in newborns and infants. J Clin Pharmacol. 2001;41(9):927–34.
Bartelink IH, Rademaker CM, Schobben AF, van den Anker JN. Guidelines on paediatric dosing on the basis of developmental physiology and pharmacokinetic considerations. Clin Pharmacokinet. 2006;45(11):1077–97.
George I, Mekahli D, Rayyan M, Levtchenko E, Allegaert K. Postnatal trends in creatinemia and its covariates in extremely low birth weight (ELBW) neonates. Pediatr Nephrol. 2011;26(10):1843–9.
Brendel K, Dartois C, Comets E, Lemenuel-Diot A, Laveille C, Tranchand B, et al. Are population pharmacokinetic and/or pharmacodynamic models adequately evaluated? A survey of the literature from 2002 to 2004. Clin Pharmacokinet. 2007;46(3):221–34.
Comets E, Brendel K, Mentre F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Prog Biomed. 2008;90(2):154–66.
Pacifici GM, Allegaert K. Clinical pharmacokinetics of vancomycin in the neonate: a review. Clinics (Sao Paulo). 2012;67(7):831–7.
Kimura T, Sunakawa K, Matsuura N, Kubo H, Shimada S, Yago K. Population pharmacokinetics of arbekacin, vancomycin, and panipenem in neonates. Antimicrob Agents Chemother. 2004;48(4):1159–67.
Jamei M, Dickinson GL, Rostami-Hodjegan A. A framework for assessing inter-individual variability in pharmacokinetics using virtual human populations and integrating general knowledge of physical chemistry, biology, anatomy, physiology and genetics: a tale of ‘bottom-up’ vs ‘top-down’ recognition of covariates. Drug Metab Pharmacokinet. 2009;24(1):53–75.
Johnson TN, Rostami-Hodjegan A. Resurgence in the use of physiologically based pharmacokinetic models in pediatric clinical pharmacology: parallel shift in incorporating the knowledge of biological elements and increased applicability to drug development and clinical practice. Paediatr Anaesth. 2011;21(3):291–301.
Allegaert K, Smits A, van den Anker JN. Physiologically based pharmacokinetic modeling in pediatric drug development: a clinician’s request for a more integrated approach. J Biomed Biotechnol. 2012;103763.
ACKNOWLEDGMENTS AND DISCLOSURES
This study was performed within the framework of Top Institute Pharma project number D2-104. The clinical research of Karel Allegaert is supported by the Fund for Scientific Research, Flanders (Clinical Fellowship 1800214N) and has been supported by an IWT-SBO project (130033). The clinical research of J. van den Anker is supported by NIH grants (R01HD060543, K24DA027992, R01HD048689, U54HD071601) and FP7 grants TINN (223614), TINN2 (260908), NEUROSIS (223060), and GRIP (261060). The authors also would like to thank LAP&P Consultants for their technical support with NONMEM.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplement I
(DOC 49 kb)
Supplement II
(DOC 52 kb)
Rights and permissions
About this article
Cite this article
De Cock, R.F.W., Allegaert, K., Sherwin, C.M.T. et al. A Neonatal Amikacin Covariate Model Can Be Used to Predict Ontogeny of Other Drugs Eliminated Through Glomerular Filtration in Neonates. Pharm Res 31, 754–767 (2014). https://doi.org/10.1007/s11095-013-1197-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-1197-y