Abstract
Purpose
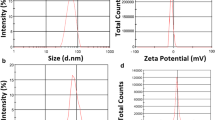
The purpose of the study was to produce BMN 673 loaded solid lipid nanoparticles (SLNs) to improve its therapeutic index, to minimize toxicity and to overcome homologous recombination (HR)-mediated resistance.
Methods
Firstly, BMN 673-SLNs were characterized using Nano Zeta Sizer. After treatment with different concentrations of BMN 673 and BMN 673-SLNs, cell viability of HCC1937(BRCA1−/−), HCC1937-R (BMN 673-resistant) TNBC and MCF-10A normal human mammary breast epithelial cell line was analyzed by WST-1 assay. In an attempt to assess the therapeutic synthetic lethality efficacy of SLNs formulation, cell cycle arrest, DNA damage, mRNA expression levels of PARP1, H2AFX, RAD51 and BRCA1 gene were investigated. Then, PARP, ɣH2AX, RAD51 and BRCA1 protein expression and nuclear localization were analyzed by western blot and immunofluorescence analysis.
Results
When compared with BMN 673, BMN 673-SLNs showed remarkably a decrease in HCC1937 and HCC1937-R cells with less damage to MCF-10A cells. BMN 673-SLNs significantly induced toxicity through double-stranded DNA breaks, G2/M cell cycle arrest and PARP cleavage in TNBC cells. Additionally, BMN 673-resistance was mediated by miR-107, miR-193b and miR-1255b targeting BRCA1 and RAD51 in HCC1937 and HCC1937-R cells. However, BMN 673-SLNs treatment could overcome HR-mediated resistance in TNBC cells.
Conclusions
As a result, our findings suggest that SLNs formulation strongly provides a synthetic lethal therapeutic potential in BRCA1 mutated sensitive and resistant TNBC cells.












Similar content being viewed by others
Abbreviations
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- dsDNA:
-
Double-strand DNA breaks
- FBS:
-
Fetal bovine serum
- GMS:
-
Glycerol monostearate
- HR:
-
Homologous recombination
- SLNs:
-
Solid lipid nanoparticles
- TNBC:
-
Triple negative breast cancer
References
Chan DA, Giaccia AJ. Harnessing synthetic lethal interactions in anticancer drug discovery. Nat rev drug Discov [internet]. Nat Publ Group. 2011;10:351–64.
Burgess M, Puhalla S. BRCA 1/2-mutation related and sporadic breast and ovarian cancers: more alike than different. Front Oncol. 2014;4:19.
Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8:193–204.
Helleday T. The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings. Mol Oncol Elsevier BV. 2011;5:387–93.
Arnaudeau C, Lundin C, Helleday T. DNA double-strand breaks associated with replication forks are predominantly repaired by homologous recombination involving an exchange mechanism in mammalian cells. J Mol Biol. 2001;307:1235–45.
Livraghi L, Garber JE. PARP inhibitors in the management of breast cancer: current data and future prospects. BMC med [internet]. BMC Med. 2015;13:188.
Ashworth A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol. 2008;26:3785–90.
Lord CJ, Tutt ANJ, Ashworth A, Synthetic Lethality, Cancer Therapy. Lessons learned from the development of PARP inhibitors. Annu Rev Med [Internet]. 2015;66:455–70.
Anders CK, Winer EP, Ford JM, Dent R, Silver DP, Sledge GW, et al. Poly(ADP-ribose) polymerase inhibition: “targeted” therapy for triple-negative breast cancer. Clin Cancer Res [Internet]. 2010;16:4702–10.
Reis-Filho JS, Ph D, Chacón RD, Costanzo M V, Dawood S, Foulkes WD, et al. Triple-negative breast cancer. Breast Cancer Res [Internet]. 2010;12 Suppl 2:S3.
Hiller DJ, Chu QD. Current status of poly(ADP-ribose) polymerase inhibitors as novel therapeutic agents for triple-negative breast Cancer. Int J Breast Cancer [Internet]. 2012;829315:2012.
Audeh MW. Novel treatment strategies in triple-negative breast cancer: specific role of poly(adenosine diphosphate-ribose) polymerase inhibition. 2014;307–316.
Stevens KN, Vachon CM, Couch FJ. Genetic susceptibility to triple-negative breast Cancer. Cancer Res [Internet]. 2013;73:2025–30.
Mayer IA, Abramson VG, Lehmann BD, Pietenpol JA. New strategies for triple-negative breast cancer-deciphering the heterogeneity. Clin Cancer Res [Internet]. 2014;20:782–90.
Barber LJ, Sandhu S, Chen L, Campbell J, Kozarewa I, Fenwick K, et al. Secondary mutations in BRCA2 associated with clinical resistance to a PARP inhibitor. J Pathol. 2013;229:422–9.
Eskiler GG, Cecener G, Egeli U, Tunca B. Triple negative breast cancer : new therapeutic approaches and BRCA status. APMIS. 2018;126:371–9.
Shen Y, Rehman FL, Feng Y, Boshuizen J, Bajrami I, Elliott R, et al. BMN 673, a novel and highly potent PARP1/2 inhibitor for the treatment of human cancers with DNA repair deficiency. Clin Cancer Res [Internet]. 2013;19:5003–5015.
Murai J, Huang S-YN, Renaud A, Zhang Y, Ji J, Takeda S, et al. Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol Cancer Ther [Internet]. 2014;13:433–43.
Cardnell RJ, Feng Y, Diao L, Fan Y-H, Masrorpour F, Wang J, et al. Proteomic markers of DNA repair and PI3K pathway activation predict response to the PARP inhibitor BMN 673 in small cell lung Cancer. Clin Cancer Res [Internet]. 2013;19:6322–8.
Wainberg ZA, de Bono JS, Mina L, Sachdev J, Byers LA, Chugh R, et al. Update on first-in-man trial of novel oral PARP inhibitor BMN 673 in patients with solid tumors. Mol Cancer Ther [Internet]. 2013;12:C295–5.
de Bono JS, et al. First-in-human trial of novel oral PARP inhibitor BMN 673 in patients with solid tumors. ASCO [internet]. 2013. P. In: Abstract no; 2580.
Lord CJ, Ashworth A. Mechanisms of resistance to therapies targeting BRCA-mutant cancers. Nat Med [Internet]. 2013;19:1381–8.
Montoni A, Robu M, Pouliot ??milie, Shah GM. Resistance to PARP-inhibitors in cancer therapy. Front Pharmacol 2013;4 FEB:1–7.
Gottipati P, Vischioni B, Schultz N, Solomons J, Bryant HE, Djureinovic T, et al. Poly(ADP-ribose) polymerase is hyperactivated in homologous recombination-defective cells. Cancer Res. 2010;70:5389–98.
Choi YE, Meghani K, Brault M-EE, Leclerc L, He YJ, Day TA, et al. Platinum and PARP inhibitor resistance due to overexpression of MicroRNA-622 in BRCA1-mutant ovarian Cancer. Cell Rep. 2016;14:429–39.
Choi YE, Pan Y, Park E, Konstantinopoulos PA, De S, D’Andrea AD, et al. MicroRNAs down-regulate homologous recombination in the G1 phase of cycling cells to maintain genomic stability. elife. 2014;2014:1–21.
Huang J-W, Wang Y, Dhillon KK, Calses P, Villegas E, Mitchell PS, et al. Systematic screen identifies miRNAs that target RAD51 and RAD51D to enhance chemosensitivity. Mol Cancer Res [Internet]. 2013;11:1564–73.
Weiss J. Solid lipid nanoparticles: a new and effective delivery system for bioactives in foods. 2009;
Loxley A. Solid lipid nanoparticles for the delivery of pharmaceutical actives. Drug Deliv Technol. 2009;9:32–7.
Mäder K, Mehnert W. Solid lipid nanoparticles-concepts, procedures, and physicochemical aspects. In: Nastruzzi C, editor. Lipospheres drug targets Deliv. Florida: CRC Press; 2005. p. 1–23.
Müller RH, Mehnert W, Lucks JS, Ruhl D. Solid lipid nanoparticles (SLN) – an alternative colloidal carrier system for controlled drug delivery. Eur J Pharm Biopharm. 1995;41:62–9.
Üner M, Yener G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspective. Int J Nanomedicine. 2007;2:289–300.
Nair R, Kumar KSA, Priya KV, Sevukarajan M. Recent Advances in Solid Lipid Nanoparticle Based Drug Delivery Systems. 2011;3:368–84.
Dineshkumar B, Krishnakumar K, Bhatt AR, John A, Paul D, Suresh JCS. Current Research in Drug Targeting Solid Lipid Nanoparticles : Investigation in Cancer Cell Lines – A Review. 2012;2:1–3.
Guney Eskiler G, Dikmen G, Genc L. Nano-based drug delivery system. In: Naik J, editor. Nano Based Drug Deliv. Zagreb, Croia: IAPC Publishing; 2015. p. 89–133.
Muller RH, Keck CM. Challenges and solutions for the delivery of biotech drugs - a review of drug nanocrystal technology and lipid nanoparticles. J Biotechnol. 2004;113:151–70.
Postel-Vinay S, Bajrami I, Friboulet L, Elliott R, Fontebasso Y, Dorvault N, et al. A high-throughput screen identifies PARP1/2 inhibitors as a potential therapy for ERCC1-deficient non-small cell lung cancer. Oncogene [Internet]. 2013;32:5377–87.
Koppensteiner R, Samartzis EP, Noske A, Von Teichman A, Dedes I, Gwerder M, et al. Effect of MRE11 loss on PARP-inhibitor sensitivity in endometrial cancer in vitro. PLoS One. 2014;9:e100041.
Herriott A, Tudhope SJ, Junge G, Rodrigues N, Patterson MJ, Woodhouse L, et al. PARP1 expression, activity and ex vivo sensitivity to the PARP inhibitor, talazoparib (BMN 673), in chronic lymphocytic leukaemia. Oncotarget [Internet]. 2015;6:43978–91.
Huang J, Wang L, Cong Z, Amoozgar Z, Kiner E, Xing D, et al. The PARP1 inhibitor BMN 673 exhibits immunoregulatory effects in a Brca1−/− murine model of ovarian cancer. Biochem Biophys Res Commun [Internet]. Elsevier Ltd; 2015;463:551–556.
Wang B, Chu D, Feng Y, Shen Y, Aoyagi-Scharber M, Post LE. Discovery and Characterization of (8 S ,9 R )-5-Fluoro-8-(4-fluorophenyl)-9-(1-methyl-1 H -1,2,4-triazol-5-yl)-2,7,8,9-tetrahydro-3 H -pyrido[4,3,2-de]phthalazin-3-one (BMN 673, Talazoparib), a Novel, Highly potent, and orally efficacious poly(ADP-ribose). J Med Chem [Internet]. 2016;59:335–57.
Smith MA, Reynolds CP, Kang MH, Kolb EA, Gorlick R, Carol H, et al. Synergistic activity of PARP inhibition by Talazoparib (BMN 673) with Temozolomide in pediatric Cancer models in the pediatric preclinical testing program. Clin Cancer Res [Internet]. 2015;21:819–32.
Mulrane L, McGee SF, Gallagher WM, O’Connor DP. miRNA dysregulation in breast cancer. Cancer Res. 2013;73:6554–62.
Wan G, Mathur R, Hu X, Zhang X, Lu X. miRNA response to DNA damage. Trends Biochem Sci [Internet]. 2011;36:478–84.
Zheng T, Wang J, Chen X, Liu L. Role of microRNA in anticancer drug resistance. Int J Cancer. 2010;126:2–10.
Eskiler GG, Cecener G, Dikmen G, Genc L, Egeli U. The effect of solid lipid nanoparticles on tamoxifen - resistant breast cancer. Int J Pharm Pharm Sci 2016;8(2):43–46.
Güney G, Kutlu HM, Genç L. Preparation and characterization of ascorbic acid loaded solid lipid nanoparticles and investigation of their apoptotic effects. Colloids Surfaces B Biointerfaces. 2014;121:270–80.
Guney Eskiler G, Cecener G, Dikmen G, Egeli U, Tunca B. Solid lipid nanoparticles: reversal of tamoxifen resistance in breast cancer [Internet]. Eur. J. Pharm. Sci. 2018:73–88.
Yusuf R, Frenkel K. Morphologic transformation of human breast epithelial cells MCF-10A: dependence on an oxidative microenvironment and estrogen/epidermal growth factor receptors. Cancer Cell Int. 2010;10:15–20.
Qu Y, Han B, Yu Y, Yao W, Bose S, Karlan BY, et al. Evaluation of MCF10A as a reliable model for normal human mammary epithelial cells. PLoS One. 2015;10:1–16.
Johnson N, Johnson SF, Yao W, Li Y-C, Choi Y-E, Bernhardy AJ, et al. Stabilization of mutant BRCA1 protein confers PARP inhibitor and platinum resistance. Proc Natl Acad Sci U S A [Internet]. 2013;110:17041–6.
Nakagawa Y, Sedukhina AS, Okamoto N, Nagasawa S. NF- κ B signaling mediates acquired resistance after PARP inhibition. Oncotarget. 2015;6:3825–39.
Guney Eskiler G. Investigation of the role of PARP inhibitors loaded solid lipid nanoparticles on overcoming drug resistance mechanisms in triple negative breast cancer treatment. PhD Thesis, Bursa: Uludag University; 2017.
Weston VJ, Oldreive CE, Skowronska A, Oscier DG, Pratt G, Dyer MJS, et al. The PARP inhibitor olaparib induces significant killing of ATM -deficient lymphoid tumor cells in vitro and in vivo. Blood. 2010;116:4578–87.
De Lorenzo SB, Patel AG, Hurley RM, Kaufmann SH. The elephant and the blind men: making sense of PARP inhibitors in homologous recombination deficient tumor cells. Front Oncol [Internet]. 2013;3:228.
Hong R, Ma F, Zhang W, Yu X, Li Q. Luo Y, et al. 53BP1 depletion causes PARP inhibitor resistance in ATM-deficient breast cancer cells. BMC Cancer [internet]. BMC Cancer. 2016;16:725.
Gilardini Montani MS, Prodosmo A, Stagni V, Merli D, Monteonofrio L, Gatti V, et al. ATM-depletion in breast cancer cells confers sensitivity to PARP inhibition. J Exp Clin Cancer Res [Internet]. 2013;32:95.
Fojo T, Bates S. Mechanisms of resistance to PARP inhibitors-three and counting. Cancer Discov. 2013;3:20–3.
Chiarugi A. A snapshot of chemoresistance to PARP inhibitors. Trends Pharmacol Sci [internet]. Elsevier Ltd. 2012;33:42–8.
Bitler BG, Watson ZL, Wheeler LJ, Behbakht K. PARP inhibitors: clinical utility and possibilities of overcoming resistance. Gynecol Oncol. 2017;147(61):695–704.
Yalon M, Tuval-Kochen L, Castel D, Moshe I, Mazal I. Cohen O, et al. overcoming resistance of cancer cells to PARP-1 inhibitors with three different drug combinations. PLoS One. 2016;11:1–20.
Sedukhina AS, Sundaramoorthy E, Hara M, Kumai T, Sato K. Beyond resistance to PARP inhibition: mechanisms and effective treatment options. Cancer Cell Microenviron [Internet]. 2015;31:14–7.
Wang Y, Krais JJ, Bernhardy AJ, Nicolas E, Cai KQ, Harrell MI, et al. RING domain–deficient BRCA1 promotes PARP inhibitor and platinum resistance. J Clin Invest [Internet]. 2016;126:3145–57.
Au WWY, Henderson BR. The BRCA1 RING and BRCT domains cooperate in targeting BRCA1 to ionizing radiation-induced nuclear foci. J Biol Chem. 2005;280:6993–7001.
Dever SM, Golding SE, Rosenberg E, Adams BR, Idowu MO, Quillin JM, et al. Mutations in the BRCT binding site of BRCA1 result in hyperrecombination. Aging (Albany NY). 2011;3:515–32.
Rodriguez JA, Au WWY, Henderson BR. Cytoplasmic mislocalization of BRCA1 caused by cancer-associated mutations in the BRCT domain. Exp Cell Res. 2004;293:14–21.
Johnson SF, Cruz C, Greifenberg AK, Dust S, Stover DG, Chi D, et al. CDK12 inhibition reverses De novo and acquired PARP inhibitor resistance in BRCA wild-type and mutated models of triple-negative breast Cancer. Cell Rep. 2016;17:2367–81.
Andrei A-Z, Hall A, Smith AL, Bascuñana C, Malina A, Connor A, et al. Increased in vitro and in vivo sensitivity of BRCA2-associated pancreatic cancer to the poly(ADP-ribose) polymerase-1/2 inhibitor BMN 673. Cancer Lett [Internet]. Elsevier Ireland Ltd; 2015;364:8–16.
Velic D, Couturier A, Ferreira M, Rodrigue A, Poirier G, Fleury F, et al. DNA damage Signalling and repair inhibitors: the long-sought-after Achilles’ heel of Cancer. Biomolecules [Internet]. 2015;5:3204–59.
Miyasaka A, Oda K, Ikeda Y, Wada-hiraike O, Kashiyama T. Enomoto A, et al. Anti-tumor activity of olaparib , a poly in cultured endometrial carcinoma cells. 2014:1–10.
Rukov JL, Shomron N. MicroRNA pharmacogenomics: post-transcriptional regulation of drug response. Trends Mol med [internet]. Elsevier Ltd. 2011;17:412–23.
Acknowledgments and Disclosures
We would like to thank Dr.Gokhan Dikmen in Central Research Laboratory, Application and Research Center (ARUM), Eskisehir Osmangazi University, who kindly provided technical support to this study. This study was supported by a grant from the Scientific Research Projects Foundation (BAP) of the Uludag University of Turkey [Project No: BUAP(T)-2015/1]. The authors declare that they have no conflicts of interest. This study represents part of the doctoral dissertation research of Gamze Guney Eskiler completed in the Department of Medical Biology at the University of Uludag.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guney Eskiler, G., Cecener, G., Egeli, U. et al. Synthetically Lethal BMN 673 (Talazoparib) Loaded Solid Lipid Nanoparticles for BRCA1 Mutant Triple Negative Breast Cancer. Pharm Res 35, 218 (2018). https://doi.org/10.1007/s11095-018-2502-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-018-2502-6