Abstract
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in China and its spread worldwide has become one of the biggest health problem due to the lack of knowledge about an effective chemotherapy. Based on the current reality of the SARS-CoV-2 pandemic, this study aimed to make a review literature about potential anti-coronavirus natural compounds guided by an in silico study. In the first step, essential oils from native species found in the Brazilian herbal medicine market and Brazilian species that have already shown antiviral potential were used as source for the literature search and compounds selection. Among these compounds, 184 showed high antiviral potential against rhinovirus or picornavirus by quantitative structure–activity relationship analysis. (E)-α-atlantone; 14-hydroxy-α-muurolene; allo-aromadendrene epoxide; amorpha-4,9-dien-2-ol; aristochene; azulenol; germacrene A; guaia-6,9-diene; hedycaryol; humulene epoxide II; α-amorphene; α-cadinene; α-calacorene and α-muurolene showed by a molecular docking study the best result for four target proteins that are essential for SARS-CoV-2 lifecycle. In addition, other parameters obtained for the selected compounds indicated low toxicity and showed good probability to achieve cell permeability and be used as a drug. These results guided the second literature search which included other species in addition to native Brazilian plants. The majority presence of any of these compounds was reported for essential oils from 45 species. In view of the few studies relating essential oils and antiviral activity, this review is important for future assays against the new coronavirus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In December 2019, for the first time the new coronavirus (SARS-CoV-2) was isolated from patients with acute pneumonia in Wuhan (China) (Guarner 2020). In February 2020, the World Health Organization (WHO) characterized this situation as a pandemic, and the new virus has already infected more people than its two predecessors, SARS-CoV (Severe acute respiratory syndrome coronavirus) and MERS-CoV (Middle East respiratory syndrome coronavirus) (Guarner 2020). In preliminary studies, the reason for the higher infectivity of the new coronavirus compared to the previous ones has been related to the greater affinity for binding to human proteins and because it is potentially more stable and able to survive higher temperatures (Chu et al. 2020; He et al. 2020).
The growing number of infections and deaths resulting from the Coronavirus Disease 2019 (COVID-19) pandemic highlights the need for an accelerated search for effective treatments. Drug repositioning, also known as redirecting, repurposing, and reprofiling, is defined as a different application of the drug than the one that it was initially produced and emerges as an effective and quick possibility to combat COVID-19 (Serafin et al. 2020). Several drugs already in use have been tested against SARS-CoV-2 and, among them, the majority drugs are antivirals (Sanders et al. 2020).
Essential oils have important pharmacologic proprieties, such as broad-spectrum antibacterial, antifungal, antiviral action and immunomodulation, in addition to few adverse effects (Raut and Karuppayil 2014; Valdivieso-Ugarte et al. 2019). Thus, essential oils show great efficacy and speed of action, and they can represent an important source for the development of COVID-19 treatments. In this way, this study aimed to evaluate the potential of compounds present in essential oils from Brazilian species. Brazil hosts more than 20% of the biodiversity in the world, with more than 46,000 species of plants, algae and fungi that were already identified and cataloged (Dutra et al. 2016; Filard et al. 2018). These numbers become even more impressive when considering that the knowledge about the country’s entire biodiversity is incomplete, and researchers are still working on building of this information (Filard et al. 2018). Therefore, Brazilian flora has a rich chemical variety that makes it a potential source for drug discovery (Valli and Bolzani 2019).
Despite all this diversity and vast use by the population, few species are licensed as pharmaceutical ingredients (Carvalho et al. 2018). This occurs because there is little published scientific and ethnopharmacological data, or protocols for quality and safety assurance for native plants (Cavalho et al. 2018). Clinical trials involving native Brazilian plants, extracts, essential oils or their active ingredients are even smaller, although there is a solid traditional use by the local population (Carvalho et al. 2018; Dutra et al. 2016). One of the reasons for the few studies involving native plant species is the strict Brazilian standards for access to biodiversity, and the lack of adequate documentation for the use and regulation of these species (Carvalho et al. 2018; Dutra et al. 2016). This fact justifies the need for further studies focused on the native Brazilian flora (Carvalho et al. 2018; Dutra et al. 2016).
Several reviews have been published about natural products as possible drugs against SARS-CoV-2. However, in the face of this pandemic for which no specific drug has yet been approved, it is essential to increasingly expand studies in search of effective treatment. Therefore, this review included essential oils from native Brazilian species with previously reported antiviral activity, as well as native species licensed in Brazil found in the herbal medicine market, aiming to involve natural products accessible to the population. These Brazilian species were used as a source of chemical diversity. In addition, in order to deepen this research, other species apart from the native ones were also investigated and indicated in the second stage of this review. An illustrative summary of the methods and results obtained can be found in Fig. 1.
Workflow scheme. The work is divided into four main parts: (1) Search of natural compounds present in essential oils from Brazilian native species; (2) QSAR analysis and selection of compounds with high antiviral potential; (3) Molecular docking analysis and selection of top 14 compounds with the highest binding energies values for papain-like protease (PLpro), 3-Chymotrypsin-like protease (3CLpro), spike glycoprotein (S protein), and RNA-dependent RNA polymerase (RdRp); (4) Search of species containing at least one of selected compounds as top 5 constituent in their essential oil
Essential oils from Brazilian native species
In the first part of the review, literature indexed in PubMed concerning natural compounds present in essential oils from native plant species licensed in Brazil was selected for the research. The species “Ananas comosus”, “Anadenanthera colubrina”, “Bacopa monieri”, “Brosimum gaudichaudii”, “Caesalpinia ferrea”, “Carapichea ipecacuanha”, “Cereus jamacaru”, “Cereus peruvianus”, “Cordia curassavica”, “Croton heliotropiifolius”, “Dorstenia aritoflia”, “Erythrina velutina”, “Erythrina verna”, “Himatanthus lancifolius”, “Lantana camara”, “Maytenus ilicifolia”, “Mikania glomerata”, “Myroxylon balsamum”, “Operculina hamiltonii”, “Passiflora alata”, “Paullinia cupana”, “Schinus terebinthifolia”, “Senna alexandrina”, “Solanum paniculatum”, “Stryphnodendron adstringens”, “Trichilia catigua”, and “Uncaria tomentosa” were used as query keywords (Carvalho et al. 2018). This research was carried out in PubMed database considering articles from January 1st 2005 to March 31th 2020. Studies related only to biological assays, review works, and manuscripts in a non-English language were excluded. The natural compounds reported in studies on chemical characterization of essential oils were tabulated and repeated references to the same compound were also excluded. The same research pattern was also used for essential oils from Brazilian species that have already shown antiviral action: “Aloysia gratissima”, “Ayapana triplinervis”, “Eupatorium patens”, “Heterothalamus alienus”, “Hyptis mutabilis”, “Lippia alba”, “Lippia origanoides”, “Ocimum campechianum”, “Pectis odorata”, and “Tessaria absinthioides” (Silva et al. 2020).
From this research, among the 27 licensed native species previously reported, seven species had already their essential oils chemically characterized and the compounds for each species are informed in the Table S1, Supplementary material. In addition, the constituents present in the essential oils evaluated as antiviral agent were also reported (Table S1, Supplementary material), except for the Pectis odorata. All compounds were considered to the in silico assays, regardless of their concentration, since Brazilian species were used as a source of chemical diversity.
These licensed species used in the Brazilian traditional medicine are: (1) Cordia curassavica, used as anti-inflammatory; (2) Croton heliotropiifolius, stimulant; (3) Lantana camara and (4) Mikania glomerata, expectorant; (5) Passiflora alata, as anxiolytic, sedative and expectorante; (6) Schinus terebinthifolia, anti-infective and healing; (7) Solanum paniculatum, cholagogue, choleretic and hepatoprotective (Carvalho et al. 2018).
Regarding antiviral activity, this action was not reported yet for essential oils from these species, but studies indicate the activity of their extracts. The aqueous extract from Lantana camara leaves has shown action against white spot syndrome virus in shrimp (Balasubramanian et al. 2007). In addition, the aqueous extract from Mikania glomerata and Solanum paniculatum leaves had antiviral activity against Suid Herpesvirus (SuHV-1) (Kaziyama et al. 2012). The hydroethanolic extract from Schinus terebinthifolia stem bark also inhibited Herpes simplex virus type 1 strains (HSV-1) (Nocchi et al. 2017). On the other hand, no antiviral action has been reported in the literature for Cordia curassavica, Croton. heliotropiifolius and Passiflora alata.
Based on the few studies of antiviral activity for licensed species, other Brazilian species that have already shown action against any virus were added to the research in order to increase the search source for compounds with potential activity against the new coronavirus. In the study performed by García et al. (2003), different essential oils were screened for virucidal property and Aloysia gratissima and Tessaria absinthioides showed potent inhibition on junin virus (JUNV) and HSV-1, whereas Eupatorium patens was active against HSV-1 and dengue virus type 2 (DEN-2). In this same way, Heterothalamus alienus essential oil was also able to inhibit JUNV, HSV-1, and DEN-2 viruses (Duschatzky et al. 2005). Inhibitory effect in the early stages of infection by other herpes virus (HSV-2) was also observed after treatment with essential oils from Hyptis mutabilis and Ocimum campechianum (Brand et al. 2016).
Recently, the outbreak of the Zika virus also raised concerns for the scientific community due to the severe disorders caused by this infection and the absence of an approved drug to combat it. However, essential oils like the one obtained from Ayapana triplinervis prove to be a potent phytocompound against this virus (Haddad et al. 2019). In addition, it is worth highlighting the great potential of essential oils extracted from Lippia genus as an antiviral agent. Lippia origanoides has already shown action against yellow fever virus and the same property was demonstrated for Lippia alba (Gómez et al. 2013; Meneses et al. 2009), which was also effective against four dengue viruses (DENV-1, DENV-2, DENV-3, and DENV-4) (Ocazionez et al. 2010). All these results show the broad-spectrum antiviral action of essential oils and, therefore, their potential should be investigated against to COVID-19.
Anti-COVID-19 potential
Although the antiviral activity of essential oils from native plant species licensed in Brazil has not been tested, they have other pharmacological properties and may have the potential to treat SARS-CoV-2, as indicated by other drug repositioning studies. In addition to drug repositioning, other alternatives can accelerate the research for COVID-19 treatments. The virtual screening (in silico) is an important tool to guide both in vitro and in vivo research, minimizing the assays cost, improving chances of finding the desired drug candidates and generating results in a shorter time (Pinzi and Rastelli 2019). In this way, the compounds reported in essential oils from these species were subjected to quantitative structure–activity relationship (QSAR) analysis using the program Prediction of Activity Spectra for Substances (PASS online). The aim of this test was to predict the antiviral potential of these compounds. Their structure was compared with substances that are active against viruses and are available in the database. The action specifically on rhinovirus and picornavirus was selected due the fact of that these two species belong to the same Coronavirus group [( +)ssRNA − Group IV] (Fernández-Miragall et al. 2009; Schrauf et al. 2009; Kim et al. 2012; Zhu et al. 2020b, c). The probabilities of each compound to be active (Pa) and inactive (Pi) were reported and the compounds with Pa–Pi ≥ 0.5 were classified as high potential (Seibert et al. 2019).
In the first step, 531 compounds were identified from research papers published that had investigated the composition of essential oils, among which 184 had high potential (Pa–Pi ≥ 0.5) against rhinovirus and/or picornavirus in the analysis performed by PASS online (Table S2, Supplementary material).
Human rhinovirus and picornavirus belong to the Picornaviridae family, which are nonenveloped viruses with a positive ssRNA genome (Schrauf et al. 2009; Fernández-Miragall et al. 2009). Although SARS-CoV-2 is a β-coronavirus and belongs to a different family, Coronaviridae, it has non-segmented positive-sense RNA, as well as previous viruses (Zhu et al. 2020a). Thus, these three virus contain the same genome classification, belonging to the same group [( +)ssRNA − Group IV].
In addition, phylogenetic analysis has demonstrated that viruses belonging to Picornaviridae and Coronaviridae families can be classified into the picornavirus-like supercluster and a same compound may have therapeutic potential targeted to multiple viruses in this supercluster (Kim et al. 2012). Furthermore, there are studies suggesting that results can be extrapolated between different viruses. Muller et al. (2018) found that the natural compound silvestrol, a flavagline isolated from plants of the genus Aglaia, presents antiviral activity against coronavirus and picornavirus. The target of silvestrol is the eIF4A helicase required to unwind stable RNA secondary structures in 5′ UTRs of capped mRNAs to create a binding platform to initiate protein synthesis (Chu et al. 2016; Hinnebusch et al. 2016). Silvestrol increase the affinity of eIF4A to RNA impairing the activity of the eIF4F complex (Sadlish et al. 2013). This translation mechanism occurs in coronavirus, such as MERS-CoV and HCoV-229E and this fact justifies Muller et al. (2018) findings. However, the authors also found silvestrol activity in picornavirus (human rhinovirus A1 and poliovirus type 1). It is known that picornavirus, although utilizing a different mechanism for viral translation (IRES-dependent mechanism), still require eIF4A helicase activity for efficient translation initiation (Bordeleau et al. 2006). Kuo et al (2009) also presented molecules that act in both groups of viruses. Those molecules act inhibiting the proteases 3Cpro and 3CLpro in picornavirus (CVB3, EV71 and RV14) and coronavirus (SARS-CoV and CoV-229E), respectively. Therefore, the potential action against rhinovirus and picornavirus were selected to perform the compound screening effective to SARS-CoV-2, since there are few studies on the new coronavirus, which would make the QSAR analysis unfeasible.
This strategy was based on non-specific antiviral properties and broad spectrum mechanisms of action of essential oils on viruses that can be related to their complex chemical composition. Morphological alteration is an example of mode of action of these natural products since they can destroy or mask the virus which affects viral attachment and influences its adsorption in the host cells (Ma and Yao 2020). However, the viral structure is very simple, being basically composed by genetic material surrounded by a protein wrap (Böhme et al. 2013). In this way, genome-related sites can be a target of essential oils but the protein inhibition is a promising alternative to treat the COVID-19 (Mori et al. 2016; Sharma and Kaur 2020). According to Wu et al. (2020), the main therapies are related to inhibition of viral life cycle and inhibition of structural proteins. Based on these data, proteins involved to maturation and infectivity of the virus such as papain-like protease (PLpro) and 3-chymotrypsin-like protease (3CLpro) as well as spike glycoprotein (S protein) and RNA-dependent RNA polymerase (RdRp) that act in the cell cycle of the virus were selected for next analysis.
Thus, the previous compounds that showed high potential against at least one of the viruses (rhinovirus or picornavirus) were subjected to molecular docking studies. This analysis was carried out by AutoDock Vina tool using PyRx software in order to understand the interaction between these compounds and the targets proteins to combat the novel coronavirus infection (Dallakyan and Olson 2015). Crystal structures of SARS-CoV-2 (2019-nCoV) PLpro (Protomer PDB ID 6W9C), main protease, also called 3CLpro (PDB ID 6Y2F), S protein (Protomer PDB ID 6VSB) and RdRp (PDB ID 6M71) were obtained from the protein database (PDB). 3D structures of the compounds were obtained by the PubChem database. The files were converted to the appropriate format (*.pdb) using the Biovia Discovery Studio software (San Diego, USA). For which target, a grid box was defined in order to comprise the entire protein. Then, AutoDock Vina algorithm was used to calculate the binding energies between the targets and the compounds in the PyRx docking tool. Drugs that were already indicated by previous studies as potential anti-COVID-19 with the same proteins as molecular targets were included as positive controls: formoterol, disulfiram. nelfinavir, prulifloxacin, hydroxychloroquine, arbidol, remdesivir and favipiravir (Arya et al. 2020; Lin et al. 2018; Qamar et al. 2020; Fantini et al. 2020; Zhu et al. 2020; Wang et al. 2020; Chen et al. 2020). The binding energies were expressed in kcal/mol and converted to inhibition constant (Ki) in µM (Table S3, Supplementary material).
Dimercaprol and mechlorethamine were chosen as negative control since they are clinically established drugs without antiviral activity (Kadioglu et al. 2020). With exception of disulfiram, the positive control drugs revealed binding energies ≤ -5.2 kcal/mol and inhibition constant ≤ 153.5 µM, while negative control drugs bound with affinities ≥ -3.3 kcal/mol and inhibition constant ≥ 3798.5 µM to the four targets (Table S3, Supplementary material). Disulfiram has a reactive sulfur group that could bind covalently to cysteine proteases as PLpro and such strong binding is not commonly accounted in docking protocols, which helps to explain such high energy binding (− 3.9 kcal/mol) when compared to others positive control drugs (Lin et al. 2018). This distinction between positive and negative controls is maintained even when the binding energy values energies are normalized by the number of heavy atoms (N) (Normalized binding energy = binding energy/N1/3) (Table S4, Supplementary material). This normalization strategy normally is employed as an attempt to mitigate the contribution of the compound size to the energy score as many scoring functions tend to favor compounds with higher molecular weight due to a larger sum of van der Waals atom pairs interactions (Pan et al. 2003; Hancock et al. 2005).
The 14 compounds that showed the best results for the four analyzed targets were: (E)-α-atlantone (1); 14-hydroxy-α-muurolene (2); allo-aromadendrene epoxide (3); amorpha-4,9-dien-2-ol (4); aristolochene (5); azulenol (6); germacrene A (7); guaia-6,9-diene (8); hedycaryol (9); humulene epoxide II (10); α-amorphene (11); α-cadinene (12); α-calacorene (13) and α-muurolene (14) (Table S3, Supplementary material) (Fig. 2). All these compounds exhibited lower binding energy values (stronger affinity) than the negative controls, even after normalization as these controls have relatively low molecular weight (Table S4, Supplementary material). When compared to the positive controls, in general, the selected compounds showed favorable binding energies, even having lower molecular weight than these reference drugs which makes them more druglike (Table S4, Supplementary material).
Most of the top 14 compounds are cyclic sesquiterpenes, except the 6 which is a cyclic monoterpene. Sesquiterpenes are among the main classes of secondary metabolites with antiviral properties (Reichling 2018). These compounds had been tested against different herpesviruses, rhinovirus and hepatitis B (Astani et al. 2011; Gu et al. 2019).
The selected compounds were identified in the essential oils from Aloysia gratissima, Cordia curassiava, Lantana camara, Lippia alba, Lippia origanoides and Schinus terebinthifolia in the first research step. Among these, the native licensed species Lantana camara has the greatest potential in which seven compounds were identified: 3, 6, 7, 8, 10, 13 and 14. In addition, Lippia alba (compounds 2, 3, 7, 9, 11 and 14) and Lippia origanoides (compounds 5, 10, 11, 12, 13 and 14) also present great potential, in which six compounds were identified. Therefore, the molecular docking results suggest that these three essential oils would have potential to inhibit SARS-CoV-2 and these natural products may be indicated for future in vitro trial studies.
The proteins analyzed by molecular docking are essential for the SARS-CoV-2 lifecycle (Sanders et al. 2020). The S protein is involved in the first step of the viral replication cycle, since it mediates the virus attachment to the surface of respiratory cells, using the angiotensin-converting enzyme-2 (ACE-2) as an entry receptor (Fantini et al. 2020). This transmembrane protein forms homotrimers protruding from the viral surface and each single protomer is made up of two functional subunits S1 and S2 (Walls et al. 2020; Wrapp et al. 2020). S1 is divided into 2 subdomains: N-terminal domain and receptor-binding domain (Wrapp et al. 2020).
The compounds 1, 2, 3, 4, 5, 6, 7, 8, 9, 11, 12 and 13 are predicted to bind to the N-terminal domain of the S1 subunit, as well as the hydroxychloroquine control. The proposed mechanism of action of the hydroxychloroquine includes the inhibition the entry step of SARS-CoV-2 (Liu et al. 2020). The binding of this drug with the N-terminal domain, which is conserved among clinical isolates worldwide, inhibits the interaction between S protein and the host cell receptor (ACE-2) (Fantini et al. 2020). Thus, bonding of the mentioned compounds to the same region of hydroxychloroquine, could inhibit the interaction with ACE-2 and consequently the entry of the virus into the cell. The binding energy (unnormalized and normalized) and inhibition constant values of these compounds indicate stronger affinity with S protein than the control hydroxychloroquine (Fig. 3a and Table S4). This drug, worldwide used for the treatment of malaria and rheumatological conditions, showed anti-SARS-CoV-2 effects both in vitro and in vivo and was recommended by several national guidelines for treatment of patients with COVID-19 (such as Belgian, Italian and Chinese guidelines) (Singh et al. 2020). However, the effectiveness of this drug has not been proven by clinical trials and its serious adverse effects especially on the heart and eyes cause concern (Zou et al. 2020). Therefore, most countries no longer recommend this drug for the treatment of COVID-19.
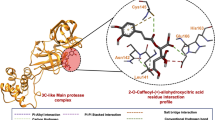
3D diagram showing the superimposed binding site of compounds from essential oils and controls with a spike glycoprotein (S protein), b papain-like protease (PLpro), c 3-Chymotrypsin-like protease (3CLpro) and d RNA-dependent RNA polymerase (RdRp). (1) (E)-α-Atlantone; (2) 14-Hydroxy-α-muurolene; (3) allo-Aromadendrene epoxide; (4) Amorpha-4,9-dien-2-ol; (5) Aristochene; (6) Azulenol; (7) Germacrene A; (8) Guaia-6,9-diene; (9) Hedycaryol; (10) Humulene epoxide II; (11) α-Amorphene; (12) α-Cadinene; (13) α-Calacorene; (14) α-Muurolene; (HCQ) hydroxychloroquine; (ARB) arbidol; (FMT) formoterol; (DSF) disulfiram; (PFX) prulifloxacin; (NFN) nelfinavir; (RDS) remdesivir and (FVP) favipiravir. aEnergy binding energy values in kcal/mol (unnormalized)
Arbidol (umifenivir) is another antiviral drug included in clinical trials that act inhibiting the virus entry. This agent is approved in China and Russia for treating influenza, SARS, and Lassa viruses and showed clinical efficacy superior to lopinavir/ritonavir in treating COVID-19 (Zhu et al. 2020b). This drug binds in the S2 subunit and it can effectively block or impede the trimerization of the S protein (Vankadari 2020). The compound 10 bound near to the binding region of arbidol and, therefore, it may present a similar mechanism of action. This compound exhibited better binding energy (unnormalized and normalized) and inhibition constant than arbidol (Fig. 3a and Table S4). On the other hand, the compound 14 bound to other region of the S2 subunit and despite the low binding energy, there is no evidence in the literature that binding at this site leads to inhibition (Fig. 3a).
In addition to S protein, PLpro and 3CLpro are enzymes also considered as promising targets, since they are cysteine proteases which mediate the proteolytic processing of maturation and infectivity of the virus (Arya et al. 2020; Qamar et al. 2020). The PLpro monomer consists of four domains: palm, thumb, and finger domains which form an extended right-hand architecture, and the N-terminal ubiquitin-like (Ubl) domain (Ratia et al. 2006). The PLpro active site is located at the bottom of the palm and thumb domains, where the control disulfiram bound (Fig. 3b). Disulfiram, an approved drug to treat alcohol dependence considered a therapeutic option for COVID-19, acts forming a covalent adduct at the active site of SARS-CoV PLpro, thus it is a competitive inhibitor (Lin et al. 2018; Li and Clercq 2020). None of the analyzed compounds indicated the same mechanism of action than disulfiram, since they did not bind to the PLpro active site.
Although they do not bind to this active site, the analyzed compounds can interfere with the PLpro functions by binding into allosteric sites with a low general inhibition constant (< 92.5 uM) (Fig. 3b), which reinforces the potential of these selected compounds as anti-coronavirus agent. The compounds 2, 4, 5, 7, 8, 10, 11 and 12 bound between the Ubl and thumb domains. The compounds 9 and 13 bound only in Ubl (Fig. 3b). The Ubl domain is not required for PLpro catalytic activity, but it is necessary for its interferon (IFN) antagonist activity, which is one viral strategy to disable the host’s innate immune response (Frieman et al. 2009). Another PLpro function involved in modulating the innate immune response is deubiquitinating. PLpro recognizes and cleaves ubiquitin, a protein that regulates a number of cellular pathways, including many processes associated with combating viral infection (Ratia et al. 2014). The region between the palm and fingers domains is important for this ubiquitin recognition (Ratia et al. 2014). The compounds 1, 3, 6 and 14 mainly bound in this region, as well as the formoterol control (Fig. 3b). Formoterol is a bronchodilator used in the management of chronic obstructive pulmonary disease (COPD) and asthma, and it has shown potential to inhibit the viral PLpro activity (Arya et al. 2020). Formoterol may reduce human coronavirus replication by inhibiting receptor expression and/or endosomal function and modulating airway inflammation after infection (Yamaya et al. 2020).
Along with PLpro, 3CLpro is essential for processing the polyproteins that are translated from the viral RNA and its inhibitors are unlikely to be toxic, since no human protease with similar cleavage specificity is known (Zhang et al. 2020). The protomer of this enzyme is divided in the domains I, II and III (Zhang et al. 2020). The compound 13 interacts with one of the amino acid residues of the catalytic site (HIS41, domain I), which might interfere with substrate entering (Fig. 3c) (Zhang et al. 2020). Binding to the 3CLpro active site, this compound may have an mechanism of action similar to ritonavir and liponavir, two anti-HIV drugs, which have been reported to be active against SARS and MERS (Nutho et al. 2020).
Others compounds (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 and 14) were predicted to bind in the region between domains II and III, important for 3CLpro dimer formation (Zhang et al. 2020). Similarly, the controls nelfinavir and prulifloxacin, considered the best drugs targeting SARS-CoV-2 3CLpro that could be used for the treatment of COVID-19, do not interact either with the 3CLpro catalytic site residues (Qamar et al. 2020). They bound between domains II and III, as well as the most of the analyzed compounds (Fig. 3c).
In relation to the mechanisms of action of these positive controls, experiments demonstrated that nelfinavir, a very safe and widely prescribed drug in the treatment of HIV-infected patients, exerts its effect not at the entry step, but at the post-entry step of SARS-CoV infection, involving partial 3CLpro block (Yamamoto et al. 2004). The nelfinavir effectiveness in inhibiting SARS-CoV-2 replication in vitro was higher than nine HIV-1 protease inhibitors including lopinavir (Yamamoto et al. 2020). Similar to nelfinavir, prulifloxacin also inhibits the 3CLpro SARS-CoV-2 interrupting the dimer formation (Li et al. 2020). The compounds 1, 3, 6, 7, 8, 9, 10, 12 and 14, probably have the same mechanism of action than nelfinavir and prulifloxacin, since they bound to the same region. None of the analyzed compounds showed binding energy and inhibition constant values lower than these positive controls (Fig. 3c). However, after binding energy normalization, compounds 2, 4, 5, 6, 7, 11, 12, 13 showed to be more promising than these reference drugs, because their normalized binding energy overcome it due to their lower molecular weight (Table S4, Supplementary material).
The enzyme RdRp (also known as nsp12) catalyzes the synthesis of viral RNA and plays a central role in the replication and transcription viral cycle. Thus, RdRp can also be target to SARS-CoV-2 therapy as demonstrated in treatment with remdesivir and favipiravir (Wang et al. 2020; Chen et al. 2020). The structure of the RdRp contains a polymerase domain (fingers, palm and thumb domains) and a nidovirus-unique N-terminal extension domain (NiRAN). These two domains are connected by an interface domain (Gao et al. 2020).
The compounds 1, 3, 5, 6, 7, 12 and 13 were predicted to bind in the polymerase domain: 1 is located in the RdRp active site, in the palm domain; 3 and 6 in the interface domain and 5, 7, 12 and 13 in the fingers domain (Fig. 3d). The binding to this polymerase domain might interfere with the viral RNA synthesis (Velthuis et al. 2014). On the other hand, the compounds 2, 4, 8, 9, 10, 11 and 14 were predicted to bind to the RdRp NiRAN domain (Fig. 3d). This domain is not part of the RdRp catalytic site, but it is essential for the virus due to potential functions that may include nucleic acid ligation, mRNA capping and protein-primed RNA synthesis (Lehmann et al. 2015).
The compound 1 did not show binding energy stronger than the controls, but it bound in the same region than remdesivir. Thus, the compound 1 probably has a mechanism of action similar to the control remdesivir, since it also bounds in the RdRp active site, which can inhibit the virus by synthesis inhibiting of viral nucleic acid (Wu et al. 2020). Remdesivir has broad-spectrum activity against members of several virus families (Ebola, SARS-CoV and MERS-CoV) and showed clinical improvement in 68% of patients with severe COVID-19 in a clinical study (Grein et al. 2020).
The compounds 5, 7, 12 and 13 exhibited binding energy (unnormalized and normalized) and inhibition constant values lower than favipiravir and they bound near to the binding region of this control, suggesting a similar mechanism of action (Fig. 3d). Favipiravir (T-705), a guanine analogue approved for influenza treatment, can effectively inhibit the viral RdRp and an in vitro study reported its activity against SARS-CoV-2 (Wang et al. 2020; Li and Clercq 2020). One clinical trial already reported that among patients with COVID-19, favipiravir significantly improved the latency for relief of pyrexia and cough when compared to arbidol (Chen et al. 2020).
Off-target, drug-like and toxicity predictions
During the new drugs discovery, in addition to the interest targets, it is also important to evaluate the possibly off-targets bindings. Off-target binding comprises the binding of a compound to a protein other than its primary target of therapeutic interest, which can lead to side-effects (Xie et al. 2011). The selected compounds 1–14 were submitted to SwissTargetPrediction, one of the standard tools of computational off-target prediction methods with 2092 Homo sapiens targets (Daina et al. 2019). The results indicated a low probability (≤ 16%) of affinity with the targets for the compounds, indicating a low potential of off-target competition, except for the compound 2. This compound showed 33% of probability of interaction with LXR-alpha (Table S5, Supplementary material). The LXR-alpha (nuclear liver X receptor alpha) is involved in the cholesterol metabolism and lipid biosynthesis. In addition, it is a regulator of inflammatory cytokines and suppressor of hepatic glucose production, being a potential drug target for treatment of diabetes (Steffensen and Gustafssonet 2004). However, the anti-diabetes effect of the compound 2 is not yet reported in the literature and its probability of affinity (33%) can be considered low.
Absorption, distribution, metabolism, excretion and toxicity (ADMET) profile has a great importance in drug discovery and development. Focusing on off-target actions, the selected compounds 1–14 were evaluated for their possible toxic effects (cardiotoxic, hepatotoxic and mutagenic) through the web-based tool ADMETlab (Dong et al. 2018). The inhibition of the hERG potassium channel may result in QT interval prolongation and causes severe cardiac side effects, which is the foremost problem in clinical studies of drug candidates (Wang et al. 2012). Regarding hepatotoxicity, drug-induced liver injury is a major cause for drug withdrawal from the market (Chen et al. 2011). Ames test, the most widely used assay for testing the mutagenicity (Xu et al. 2012), was used for this parameter prediction. Interestingly, compounds 1–14 were not highly likely for these important toxicity endpoints (except hepatotoxicity for compound 4), forecasting their security (Table S6, Supplementary material). Several in vivo studies have already demonstrated that many essential oils can be used safely (Altaei 2012; Hoff Brait et al. 2015; Cruz et al. 2017; Mishra et al. 2018). Furthermore, clinical trials have confirmed the good tolerability of essential oils use for humans (Lillehei and Halcon 2014; Hammer 2015; Han et al. 2017). Although the compound 4 has presented a prediction for hepatotoxicity, it is one constituent of Aloysia gratissima essential oil which did not cause side effects on fish when used as an anesthetic (Benovit et al. 2015).
However, it is worth highlight that some essential oil constituents can be allergens (Groot and Schmidt 2016). Therefore, in addition to the major toxicological endpoints of concern, such as acute toxicity genetic toxicity, carcinogenicity, reproductive toxicity, and developmental toxicity, allergenic potential tests must be carried out in order to determine safe doses and routes of administration before the clinical use.
In addition, the drug-likeness based on Lipinski’s rule was also predicted, on ADMETlab platform. According to Lipinski’s rule, a molecule should present molecular weight ≤ 500 Dalton, number of H-bond donors ≤ 5, number of H-bond acceptors ≤ 10 and LogP ≤ 5 to be considered as an ideal drug molecule (Lipinski 2004). The results indicated that none of 1 to 14 compounds violated the Lipinski’s parameters (except LogP for compound 7), showing good probability to achieve cell permeability and be considered as a drug candidate (Table S6, Supplementary material).
Potential anti-COVID-19 essential oils
All considered, the in silico results point out to the potential of the compounds 1–14 to be explored as anti-coronavirus agent. Essential oils are complex mixtures and this chemical diversity guarantees their different therapeutic properties. However, the major compounds, usually consisted by one to five substances, are mainly responsible for their biological effects (Bakkali et al. 2008). Based on this prediction, the second research step was based in the selection of essential oils that presented at least one of these compounds as major (at the top five) constituents in order to indicate possible natural products for future in vitro and in vivo trials against COVID-19.
This search was performed at PubMed literature on these natural compounds and potential essential oils in which they were present (Query keywords: “(E)-α-Atlantone”; “14-Hydroxy-α-muurolene”; “allo-Aromadendrene epoxide”; “Amorpha-4,9-dien-2-ol”; “Aristochene”; “Azulenol”; “Germacrene A”; “Guaia-6,9-diene”; “Hedycaryol”; “Humulene epoxide II”; “α-Amorphene”; “α-Cadinene”; “α-Calacorene”; “α-Muurolene” and “essential oil”). In addition, synonymous for these compounds described in PubChem were used in parallel as a search query keyword.
According to Table 1, 45 species were selected and it is worth highlighting that the Athanasia brownii, Calamintha nepeta, Callicarpa Americana, Chamecyparis formosensis, Cistus creticus, Cymbopogon validus, Myrtus communis, Pelargonium graveolens, Piper aduncum, Psidium guajava, Rydingia michauxii, Seseli rigidum, Tetradenia riparia, Teucrium montanum, Teucrium persicum, Ugni myricoides, Vernonia cinerea and Zingiber zerumbet species presented more than one of the compounds of interest in their constitution.
Among the species selected, antiviral potential has already been reported for Baccharis dracunculifolia. This species is a native plant from Brazil and it is important in the green propolis production. Study performed by Búfalo et al. (2009) showed a low viral (poliovirus type 1) quantification by real-time PCR after treatment with Baccharis dracunculifolia essential oil. In addition, this species has been considered safe, since it showed no cytotoxicity against HEp-2 cells, as well as it was classified as category 5 through in vivo toxicity assays by Globally Harmonized Classification System (GHS) for Chemical Substances and Mixtures (Búfalo et al. 2009, 2010; Massignani et al. 2009). Thus, Baccharis dracunculifolia essential oil stands out as a potential alternative for the treatment of COVID-19.
Additionally, as reviewed by Silva et al (2020), compounds 4 and 14 have already been identified as major compounds in essential oils with antiviral activity. Santolina insularis essential oil (12.7% of compound 4) presented high activity against herpes simplex type 1 and type 2, accompanied by low cytotoxicity (Logu et al. 2000). Fortunella margarita essential oil (10.3% of compound 14 in leaves and 5.5% in fruits) presented activity against avian influenza-A virus (Ibrahim et al. 2015).
Essential oils in clinical trials
Essential oils have been shown to be effective in clinical trials against respiratory virus infections. A double blind randomized controlled trial using essential oils from different species (Coridothymus capitatus, Origanum dictammus and Salvia fruticosa) to treat upper respiratory tract infection was performed by Duijker et al. (2015). Among the virus-positive patients, human rhinovirus, H1N1 influenza, human metapneumovirus and human coronavirus strains were identified by specific RT-qPCR. The severity and duration of symptoms of these patients were lower in the treated group compared to placebo group and the use of the essential oils was safe, since hepatic or renal function showed no changes. In this same way, randomized study performed by Shayeganmehr et al. (2018) showed that Zataria multiflora essential oil reduced H9N2 influenza replication, as well as clinical symptoms.
Regarding non-respiratory virus infections, double-blind randomized trial was performed to confirm the action of Myrtus communis against human papillomavirus (HPV). Herbal vaginal suppositories containing 0.5% of myrtle essential oil were prescribed for the intervention group and showed greater number of negative HPV tests (92.6%) after treatment when compared to the placebo group (62.6%). In addition, an improvement in the size of the cervical lesion was also observed (Nikakhtar et al. 2018).
In a controlled clinical trial to evaluate the reducing of HSV-1 virus (Herpes simplex virus, Type 1) found in saliva, infected patients were treated with a commercially available mouth rinse or sterile water as control group. The antiseptic was composed of eucalyptol, thymol, and menthol terpenes, which are found in different essential oils. The samples were analyzed at different times (30 s, 30 min and 60 min) after the treatments and it was possible to observe that the group containing terpenes was efficient at the first time, since viruses were not detected after 30 s, whereas for the group containing only water, the virus concentration was not changed (Meiller et al. 2005). Melaleuca alternifolia essential oil has also shown activity against HSV and a randomized, placebo-controlled, investigator-blinded study was performed to evaluate its efficacy. The results showed that patients treated with oil gel (6% tea tree essential oil) have median time to re-epithelialization and median duration of culture positivity (9 days and 3 days, respectively) lower than placebo group (12.5 days and 4 days, respectively) (Carson et al. 2001).
All these data indicate some benefit of essential oil for the treatment of virus infections; however, studies relating to this purpose (essential oils and clinical trials) still represent a small portion among the approach of new viral agents. The results presented in this work reinforce the antiviral potential of compounds found in essential oils and direct the species reported in the Table 1 for in vitro and in vivo assays. On the other hand, the low performance and the difficulty of isolating these components justify the use of essential oils instead of just one active compound. In addition, the complex chemical composition of essential oils allows the occurrence of a synergistic effect between their components and, consequently, greater action against viruses, as well as decreases the probability of developing resistant strains (Schnitzler 2019).
Conclusions and future perspectives
Therefore, this review shows that essential oils have potential to inhibit SARS-CoV-2 and, based on their physical–chemical characteristics, their administration may be an alternative to treat and control the COVID-19 symptoms. Furthermore, this study provides valuable information on which essential oils should be prioritized for in vitro and in vivo studies in order to confirm these results and afford a potential drug in the fight against COVID-19.
In addition, the few studies referring to antiviral potential of essential oils should be highlighted, since in a PubMed literature search less than 0.08% of works identified using the antiviral as query keyword also referred to essential oils (“antiviral” “essential oil”). This reality reinforces the importance of the present work and encourages new studies to evaluate the action of essential oils against dangerous classes of viruses.
Another important point is that the essential oils production is affected by several biotic and abiotic factors, and the qualitative and quantitative constitution of these metabolites can be altered in the same species, changed its biological effect (Gobbo-Neto and Lopes 2007). In this same way, many essential oils exist in the form of several different chemotypes and for that it is important to ensure the correct identification of the natural product. Thus, we suggest that essential oils should be chemically characterized before screening biological studies to confirm the presence of the compounds indicated (1–14) in our research. In addition, we also believe that this work can be used as a reference in the research and evaluation of other oils that have not been identified in the PubMed literature or that have not yet been reported.
Supplementary material
Tables of the compounds reported in the species and QSAR, molecular docking, off-target, drug-like and toxicity predictions.
References
Afoulous S, Ferhout H, Raoelison EG, Valentin A, Moukarzel B, Couderc F, Bouajila J (2011) Helichrysum gymnocephalum essential oil: chemical composition and cytotoxic, antimalarial and antioxidant activities, attribution of the activity origin by correlations. Molecules 16(10):8273–8291
Akhbari M, Batooli H, Mozdianfard M (2012) Comparative study of composition and biological activities of SDE prepared essential oils from flowers and fruits of two Hypericum species from central Iran. Nat Prod Res 26(3):193–202
Alarcón L, Peña A, Velasco J, Baptista JG, Rojas L, Aparicio R, Usubillaga A (2015) Chemical composition and antibacterial activity of the essential oil of Ruilopezia bracteosa. Nat Prod Commun 10(4):655–656
Altaei DT (2012) Topical lavender oil for the treatment of recurrent aphthous ulceration. Am J Dent 25(1):39–43
Arya R, Das A, Prashar V, Kumar M (2020) Potential inhibitors against papain-like protease of novel coronavirus (SARS-CoV-2) from FDA approved drugs. ChemRxiv. https://doi.org/10.26434/chemrxiv.11860011
Astani A, Reichling J, Schnitzler P (2011) Screening for antiviral activities of isolated compounds from essential oils. Evid Based Complement Alternat Med 2011(253643):1–8
Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils—a review. Food Chem Toxicol 46:446–475
Balasubramanian G, Sarathi M, Kumar SR, Hameed ASS (2007) Screening the antiviral activity of Indian medicinal plants against white spot syndrome virus in shrimp. Aquaculture 263(1–4):15–19
Benovit SC, Silva LL, Salbego J, Loro VL, Mallmann CA, Baldisserotto B, Flores EM, Heinzmann BM (2015) Anesthetic activity and bio-guided fractionation of the essential oil of Aloysia gratissima (Gillies & Hook.) Tronc. in silver catfish Rhamdia quelen. An Acad Bras Cienc 87(3):1675–1689
Bisht DS, Joshi SC, Padalia RC, Mathela CS (2012) Isoiridomyrmecin rich essential oil from Nepeta erecta Benth, and its antioxidant activity. Nat Prod Res 26(1):29–35
Böhme K, Velázquez JB, Calo-Mata P, Aubourg SP (2013) Antimicrobial compounds. Antibacterial, antiviral and antifungal activity of essential oils: mechanisms and applications. Springer, Berlin, pp 51–81
Bordeleau ME, Mori A, Oberer M, Lindqvist L, Chard LS, Higa T, Belsham GJ, Wagner G, Tanaka J, Pelletier J (2006) Functional characterization of IRESes by an inhibitor of the RNA helicase eIF4A. Nat Chem Biol 2:213–220
Boué GB, Boti JB, Tonzibo ZF, Paoli M, Bighelli A (2018) New trans-β-bergamotene derivatives in the root and the flower essential oils of Cyanthillium cinereum (L.) H. Rob. from Côte d'Ivoire. Nat Prod Res 33(19):2795–2800
Brand YM, Roa-Linares VC, Betancur-Galvis LA, Durán-García DC, Stashenko E (2016) Antiviral activity of Colombian Labiatae and Verbenaceae family essential oils and monoterpenes on Human Herpes viruses. J Essent Oil Res 28(2):130–137
Búfalo MC, Figueiredo AS, De Sousa JPB, Candeias JMG, Bastos JK, Sforcin JM (2009) Anti-poliovirus activity of Baccharis dracunculifolia and propolis by cell viability determination and real-time PCR. J Appl Microbiol 107:1669–1680
Búfalo MC, Candeias JMG, Sousa JPB, Bastos JK, Sforcin JM (2010) In vitro cytotoxic activity of Baccharis dracunculifolia and propolis against HEp-2 cells. Nat Prod Res 24(18):1710–1718
Carson CF, Ashton L, Dry L, Smith DW, Riley TV (2001) Melaleuca alternifolia (tea tree) oil gel (6%) for the treatment of recurrent herpes labialis. J Antimicrob Chemother 48(3):450–451
Carvalho ACB, Lana TN, Perfeito JPS, Silveira D (2018) The Brazilian market of herbal medicinal products and the impacts of the new legislation on traditional medicines. J Ethnopharmacol 212:29–35
Casiglia S, Riccobono L, Bruno M, Rosselli S, Senatore F, Senatore F (2016) Chemical composition of the essential oil from Thapsia garganica L. (Apiaceae) grown wild in Sicily and its antimicrobial activity. Nat Prod Res 30(9):1042–1052
Chaaban A, Martins CEN, Bretanha LC, Micke GA, Carrer AR, Rosa NF, Ferreira L, Molento MB (2018) Insecticide activity of Baccharis dracunculifolia essential oil against Cochliomyia macellaria (Diptera: Calliphoridae). Nat Prod Res 32(24):2954–2958
Chaudhary A, Kaur P, Singh B, Pathania V (2009) Chemical composition of hydrodistilled and solvent volatiles extracted from woodchips of Himalayan Cedrus: Cedrus deodara (Roxb.) Loud. Nat Prod Commun 4(9):1934578X0900400920
Chen M, Vijay V, Shi Q, Liu Z, Fang H, Tong W (2011) FDA-approved drug labeling for the study of drug-induced liver injury. Drug Discov Today 16:697–703
Chen CJ, Kumar KJS, Chen YT, Tsao NW, Chien SC, Chang ST, Chu FH, Wang SY (2015) Effect of Hinoki and Meniki essential oils on human autonomic nervous system activity and mood states. Nat Prod Commun 10(7):1305–1308
Chen C, Zhang Y, Huang J, Yin P, Cheng Z, Wu J, Chen S, Zhang Y, Chen B, Lu M, Luo Y, Ju L, Zhang J, Wang X (2020) Favipiravir versus Arbidol for COVID-19: a randomized clinical trial. ChemRxiv. https://doi.org/10.1101/2020.03.17.20037432
Chizzola R (2014) Composition of the essential oil of wild grown caraway in meadows of the Vienna region (Austria). Nat Prod Commun 9(4):581–582
Chu J, Galicia-Vázquez G, Cencic R, Mills JR, Katigbak A, Porco JA Jr, Pelletier J (2016) CRISPR-mediated drug-target validation reveals selective pharmacological inhibition of the RNA helicase, eIF4A. Cell Rep 15:2340–2347
Chu H, Chan JFW, Wang Y, Yuen TTT, Chai Y, Hou Y, Zhang X (2020) Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa410
Cruz RCD, Silva SLCE, Souza IA, Gualberto SA, Carvalho KS, Santos FR, Carvalho MG (2017) Toxicological evaluation of essential oil from the leaves of Croton argyrophyllus (Euphorbiaceae) on Aedes aegypti (Diptera: Culicidae) and Mus musculus (Rodentia: Muridae). J Med Entomol 54(4):985–993
Dai DN, Thang TD, Ogunwande IA (2014) Chemical composition of essential oils from the leaves and stem barks of Vietnamese species of Polyalthia harmandii, Polyalthia jucunda and Polyalthia thorelii. Nat Prod Res 28(8):555–562
Daina A, Michielin O, Zoete V (2019) SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucl Acids Res 47(W1):W357–W364
Dallakyan S, Olson AJ (2015) Small-molecule library screening by docking with PyRx. Methods Mol Biol 1263:243–250
Demetzos C, Katerinopoulos HE, Kouvarakis A, Stratigakis N, Loukis A, Ekonomakis C, Spiliotis V, Tsaknis J (1997) Composition and antimicrobial activity of the essential oil of Cistus creticus subsp. eriocephalus. Planta Med 63(05):477–479
Ding W, Liping N, Xing H, Wei Z, Zhoua Q, Nong R, Chen J (2018) Natural products research in China from 2015 to 2016. Nat Prod Res 1–4
Dong J, Wang N, Yao Z, Zhang L, Cheng Y, Ouyang D, Lu A, Cao D (2018) ADMETlab: a platform for systematic ADMET evaluation based on a comprehensively collected ADMET database. J Cheminformatics 10:29
Duijker G, Bertsias A, Symvoulakis EK, Moschandreas J, Malliaraki N, Derdas SP, Tsikalas GK, Katerinopoulos HE, Pirintsos AS, Sourvinos G, Castanas E, Lionis C (2015) Reporting effectiveness of an extract of three traditional Cretan herbs on upper respiratory tract infection: Results from a double-blind randomized controlled trial. J Ethnopharmacol 163:157–166
Duschatzky CB, Possetto ML, Talarico LB, García CC, Michis F, Almeida NV, De Lampasona MP, Schuff C, Damonte EB (2005) Evaluation of chemical and antiviral properties of essential oils from South American plants. Antivir Chem Chemother 16(4):247–251
Dutra RC, Campos MM, Santos AR, Calixto JB (2016) Medicinal plants in Brazil: pharmacological studies, drug discovery, challenges and perspectives. Pharmacol Res 112:4–29
Fantini J, Scala C, Chahinian H, Yahi N (2020) Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents 55(5):105960
Fernández-Miragall O, Quinto SL, Martínez-Salas E (2009) Relevance of RNA structure for the activity of picornavirus IRES elements. Virus Res 139(2):172–182
Filardi FLR, Barros F, Baumgratz JFA, Bicudo CEM, Cavalcanti TB, Coelho MAN, Costa AF, Costa DP, Goldenberg R, Labiak PH, Lanna JM, Leitman P, Lohmann LG, Maia LC, Mansano VF, Morim MP, Peralta DF, Pirani JR, Prado J, Roque N, Secco RS, Stehmann JR, Sylvestre LS, Viana PL, Walter BMT, Zimbrão G, Forzza RC (2018) Brazilian Flora 2020: innovation and collaboration to meet target 1 of the Global Strategy for Plant Conservation (GSPC). Rodriguésia 69(4):1513–1527
Frieman M, Ratia K, Johnston RE, Mesecar AD, Baric RS (2009) Severe acute respiratory syndrome coronavirus papain-like protease ubiquitin-like domain and catalytic domain regulate antagonism of IRF3 and NF-κB signaling. J Virol 83(13):6689–6705
Gao Y, Yan L, Huang Y, Liu F, Zhao Y, Cao L, Wang T, Sun Q, Ming Z, Zhang L, Ge J, Zheng L, Zhang Y, Wang H, Zhu Y, Zhu C, Hu T, Hua T, Zhang B, Yang X, Li J, Yang H, Liu Z, Xu W, Guddat LW, Wang Q, Lou Z, Ra Z (2020) Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science 368(6492):779–782
García CC, Talarico L, Almeida N, Colombres S, Duschatzky C, Damonte EB (2003) Virucidal activity of essential oils from aromatic plants of San Luis. Argentina Phytother Res 17(9):1073–1075
Giuliani C, Pellegrino RM, Selvaggi R, Tani C, Tirillini B, Maleci Bini L (2017) Secretory structures and essential oil composition in Stachys officinalis (L.) Trevisan subsp. officinalis (Lamiaceae) from Italy. Nat Prod Res 31(9):1006–1013
Gobbo-Neto L, Lopes NP (2007) Plantas medicinais: fatores de influência no conteúdo de metabólitos secundários. Quím Nova 30(2):374–381
Gómez LA, Stashenko E, Ocazionez RE (2013) Comparative study on in vitro activities of citral, limonene and essential oils from Lippia citriodora and L. alba on yellow fever virus. Nat Prod Commun 8:249–252
Gooré SG, Ouattara ZA, Yapi TA, Békro YA, Tomi P, Paoli M, Tomi F (2017) Chemical composition of Ivorian Artabotrys insignis leaf oil. Combined analysis including 13C NMR, to quantify germacrene A and β-elemene. Nat Prod Res 31(15):1836–1839
Grecco SDS, Martins EGA, Girola N, de Figueiredo CR, Matsuo AL, Soares MG, Bertoldo BC, Sartorelli P, Lago JHG (2015) Chemical composition and in vitro cytotoxic effects of the essential oil from Nectandra leucantha leaves. Pharm Biol 53(1):133–137
Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure F, Nicastri E, Oda R, Yo K, Quiros-Roldan E, Studemeister A, Redinski J, Ahmed S, Bernett J, Chelliah D, Chen D, Chihara S, Cohen SH, Cunningham J, Monforte A, Ismail S, Kato H, Lapadula GL, Her E, Maeno T, Majumder S, Massari M, Mora-Rillo M, Mutoh Y, Nguyen D, Verweij E, Zoufaly A, Osinusi AO, DeZure A, Zhao Y, Zhong L, Chokkalingam A, Elboudwarej E, Telep L, Timbs L, Henne I, Sellers S, Cao H, Tan SK, Winterbourne L, Desai P, Mera R, Gaggar A, Myers RP, Brainard DM, Childs R, Flanigan T (2020) Compassionate use of remdesivir for patients with severe covid-19. N Engl J Med. https://doi.org/10.1056/NEJMoa2007016
Groot AC, Schmidt E (2016) Essential oils, part I: introduction. Dermatitis 27(2):39–42
Gu C, Yin A-P, Yuan H-Y, Yang K, Luo J, Zhan Y-J, Yang C-R, Zuo D-M, Li H-Z, Xu M (2019) New anti-HBV norbisabolane sesquiterpenes from Phyllantus acidus. Fitoterapia 137:104151
Guarner J (2020) Three emerging coronaviruses in two decades. Am J Clin Pathol 153(4):420–421
Gyesi JN, Opoku R, Borquaye LS (2019) Chemical composition, total phenolic content, and antioxidant activities of the essential oils of the leaves and fruit pulp of Annona muricata L. (Soursop) from Ghana. Biochem Res Int 2019. https://doi.org/10.1155/2019/4164576
Haddad JG, Picard M, Bénard S, Desvignes C, Desprès P, Diotel N, Kalamouni CE (2019) Ayapana triplinervis essential oil and its main component thymohydroquinone dimethyl ether inhibit zika virus at doses devoid of toxicity in Zebrafish. Molecules 24(19):3447
Hammer KA (2015) Treatment of acne with tea tree oil (melaleuca) products: a review of efficacy, tolerability and potential modes of action. Int J Antimicrob Agents 45(2):106–110
Hamrouni-Aschi K, Khouja ML, Boussaid M, Akrimi N, Toumi L (2013) Essential-oil composition of the Tunisian endemic cypress (Cupressus sempervirens L. var. numidica TRAB.). Chem Biodivers 10(6):989–1003
Han X, Gibson J, Eggett DL, Parker TL (2017) Bergamot (Citrus bergamia) essential oil inhalation improves positive feelings in the waiting room of a mental health treatment center: a pilot study. Phytother Res 31(5):812–816
Hancock CN, Macias A, Lee EK, Yu SY, Mackerell AD, Shapiro P (2005) Identification of novel extracellular signal-regulated kinase docking domain inhibitors. J Med Chem 48(14):4586–4595
He J, Tao H, Yan Y, Huang SY, Xiao Y (2020) Molecular mechanism of evolution and human infection with SARS-CoV-2. Viruses 12(4):428
Hinnebusch AG, Ivanov IP, Sonenberg N (2016) Translational control by 5′-untranslated regions of eukaryotic mRNAs. Science 352:1413–1416
Hoff Brait DR, Mattos Vaz MS, da Silva AJ, Borges de Carvalho LN, Souza de Araújo FH, Vani JM, da Silva MJ, Cardoso CA, Oliveira RJ, Negrão FJ, Kassuya CA, Arena AC (2015) Toxicological analysis and anti-inflammatory effects of essential oil from Piper vicosanum leaves. Regul Toxicol Pharmacol 73(3):699–705
Hulley IM, Vuuren SF, Sadgrove NJ, Wyk BE (2019) Antimicrobial activity of Elytropappus rhinocerotis (Asteraceae) against micro-organisms associated with foot odour and skin ailments. J Ethnopharmacol 228:92–98
Huong LT, Huong TT, Huong NT, Hung NH, Dat PT, Luong NX, Ogunwande IA (2020) Mosquito larvicidal activity of the essential oil of Zingiber collinsii against Aedes albopictus and Culex quinquefasciatus. J Oleo Sci 69(2):153–160
Ibrahim NA, El-Hawary SS, Mohammed MMD, Farid MA, Abdel-Wahed NAM, Ali MA, El-Abd EAW (2015) Chemical composition, antiviral against avian influenza (H5N1) virus and antimicrobial activities of the essential oils of the leaves and fruits of Fortunella margarita, lour. swingle, growing in Egypt. J Appl Pharm Sci 5:6–12
Joshi RK (2013) Chemical composition of the essential oil of Baccharoides lilacina from India. Nat Prod Commun 8(3):1934578X1300800331
Joshi RK (2015) GC/MS analysis of the essential oil of Vernonia cinerea. Nat Prod Commun 10(7):1934578X1501000746
Kadioglu O, Saeed M, Greten HJ, Efferth T (2020) Identification of novel compounds against three targets of SARS CoV-2 coronavirus by combined virtual screening and supervised machine learning. Bull World Health Organ. https://doi.org/10.2471/BLT.20.255943
Karami A, Dehghan-Mashtani N (2015) Composition of the essential oil of Stachys benthamiana Boiss. from the south of Iran. Nat Prod Res 29(15):1473–1476
Kaziyama VM, Fernandes MJB, Simoni IC (2012) Atividade antiviral de extratos de plantas medicinais disponíveis comercialmente frente aos herpesvírus suíno e bovino. Rev Bras Plantas Med 14(3):522–528
Khajeh M (2012) Optimisation of supercritical fluid extraction of essential oil components of Diplotaenia cachrydifolia: Box-Behnken design. Nat Prod Res 26(20):1926–1930
Kim Y, Lovell S, Tiew K, Mandadapu SR, Alliston KR, Battaile KP, Groutas WC, Chang K (2012) Broad-spectrum antivirals against 3C or 3C-like proteases of picornaviruses, noroviruses, and coronaviruses. J Virol 86(21):11754–11762
Kuo C, Liu H, Lo Y, Seong C, Lee K, Jung Y, Liang P (2009) Individual and common inhibitors of coronavirus and picornavirus main proteases. FEBS Lett 583(3):549–555
Lehmann KC, Gulyaeva A, Zevenhoven-Dobbe JC, Janssen GM, Ruben M, Overkleeft HS, Veelen PA, Samborskiy DV, Kravchenko AA, Leontovich AM, Sidorov IA, Snijder EJ, Posthuma CC, Gorbalenya AE (2015) Discovery of an essential nucleotidylating activity associated with a newly delineated conserved domain in the RNA polymerase-containing protein of all nidoviruses. Nucl Acids Res 43(17):8416–8434
Li G, De Clercq E (2020) Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov 19(3):149–150
Li Y, Zhang J, Wang N, Li H, Shi Y, Guo G, Liu K, Zeng H, Zou Q (2020) Therapeutic drugs targeting 2019-nCoV main protease by high-throughput screening. BioRxiv. https://doi.org/10.1101/2020.01.28.922922
Lillehei AS, Halcon LL (2014) A systematic review of the effect of inhaled essential oils on sleep. J Altern Complement Med 20(6):441–451
Lin PC, Lee JJ, Chang IJ (2016) Essential oils from Taiwan: chemical composition and antibacterial activity against Escherichia coli. J Food Drug Anal 24(3):464–470
Lin M, Moses DC, Hsieh C, Cheng S, Chen Y, Sun C, Chou C (2018) Disulfiram can inhibit MERS and SARS coronavirus papain-like proteases via different modes. Antiviral Res 150:155–163
Lipinski CA (2004) Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol 1(4):337–341
Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, Li Y, Hu Z, Zhong W, Wang M (2020) Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 6:16
Logu AD, Loy G, Pellerano ML, Bonsignore L, Schivo ML (2000) Inactivation of HSV-1 and HSV-2 and prevention of cell-to-cell virus spread by Santolina insularis essential oil. Antiviral Res 48(3):177–185
Ma L, Yao L (2020) Antiviral effects of plant-derived essential oils and their components: an updated review. Molecules 25(2627):1–13
Mancini E, De Martino L, Malova H, De Feo V (2013) Chemical composition and biological activities of the essential oil from Calamintha nepeta plants from the wild in Southern Italy. Nat Prod Commun 8(1):1934578X1300800134
Marčetić MD, Lakušić BS, Lakušić DV, Kovačević NN (2013) Variability of the root essential oils of Seseli rigidum Waldst. & Kit. (Apiaceae) from different populations in Serbia. Chem Biodivers 10(9):1653–1666
Massignani JJ, Lemos M, Maistro EL, Schaphauser HP, Jorge RF, Sousa JPB, Bastos JK, De Andrade SF (2009) Antiulcerogenic activity of the essential oil of Baccharis dracunculifolia on different experimental models in rats. Phytother Res 23:1355–1360
Meiller TF, Silva A, Ferreira SM, Jabra-Rizk MA, Kelley JI, DePaola LG (2005) Efficacy of listerine antiseptic in reducing viral contamination of saliva. J Clin Periodontol 32(4):341–346
Melo NID, Carvalho CED, Fracarolli L, Cunha WR, Veneziani RCS, Martins CHG, Crotti AEM (2015) Antimicrobial activity of the essential oil of Tetradenia riparia (Hochst.) Codd. (Lamiaceae) against cariogenic bacteria. Braz J Microbiol 46(2):519–525
Meneses R, Ocazionez RE, Martínez JR, Stashenko EE (2009) Inhibitory effect of essential oils obtained from plants grown in Colombia on yellow fever virus replication in vitro. Ann Clin Microbiol Antimicrob 8:8
Miri A, Monsef-Esfahani HR, Amini M, Amanzadeh Y, Hadjiakhoondi A, Hajiaghaee R, Ebrahimi A (2012) Comparative chemical composition and antioxidant properties of the essential oils and aromatic water from teucrium persicum boiss. Iran J Pharm Res 11(2):573–581
Mishra AK, Mishra A, Pragyam CP (2018) Screening of acute and sub-chronic dermal toxicity of Calendula officinalis L essential oil. Regul Toxicol Pharmacol 98:184–189
Mori K, Obossou EK, Suwa S, Miura S, Oh S, Jinbo N, Ishibashi Y, Shikamoto Y, Hosono T, Toda T (2016) Human Immunodeficiency virus type 1 (hiv-1) reverse transcriptase inhibitory effect of Cymbopogon nardus essential oil. Int J Adv Res 2:7–13
Mozaffari E, Abai MR, Khanavi M, Vatandoost H, Sedaghat MM, Moridnia A, Saber-Navaei M, Sanei-Dehkordi A, Rafi F (2014) Chemical composition, Larvicidal and repellency properties of Cionura erecta (L.) Griseb. Against malaria vector, Anopheles stephensi Liston (Diptera: Culicidae). J Art Dis 8(2):147
Müller C, Schulte FW, Lange-Grünweller K, Obermann W, Madhugiri R, Pleschka S, Ziebuhr J, Hartmann RK, Grünweller A (2018) Broad-spectrum antiviral activity of the eIF4A inhibitor silvestrol against corona- and picornaviruses. Antiviral Res 150:123–129
Nikakhtar Z, Hasanzadeh M, Hamedi SS, Najafi MN, Tavassoli AP, Feyzabadi Z, Meshkat Z, Saki A (2018) The efficacy of vaginal suppository based on myrtle in patients with cervicovaginal human papillomavirus infection: a randomized, double-blind, placebo trial. Phytother Res 32(10):2002–2008
Nocchi SR, Campanhoni MVP, Mello JCP, Dias-Filho BP, Carolho CA, Silva DB, Ueda-Nakamura T (2017) Antiviral activity of crude hydroethanolic extract from Schinus terebinthifolia against herpes simplex virus type 1. Planta Med 83(06):509–518
Nutho B, Mahalapbutr P, Hengphasatporn K, Pattaranggoon NC, Simanon N, Shigeta Y, Hannongbua S, Rungrotmongkol T (2020) Why are lopinavir and ritonavir effective against the newly emerged coronavirus 2019? Atomistic insights into the inhibitory mechanisms. Biochemistry 59(18):1769–1779
Ocazionez RE, Meneses R, Torres FÁ, Stashenko E (2010) Virucidal activity of Colombian Lippia essential oils on dengue virus replication in vitro. Mem Inst Oswaldo Cruz 105:304–309
Orav A, Stulova I, Kailas T, Müürisepp M (2004) Effect of storage on the essential oil composition of Piper nigrum L. fruits of different ripening states. J Agric Food Chem 52(9):2582–2586
Palá-Paúl J, Copeland LM, Brophy JJ, Goldsack RJ (2009) Essential oil composition of two new species of Phebalium (Rutaceae) from North-Eastern NSW, Australia. Nat Prod Commun 4(7):1934578X0900400722
Pan Y, Huang N, Cho S, MacKerell AD (2003) Consideration of molecular weight during compound selection in virtual target-based database screening. J Chem Inf Comput Sci 43(1):267–272
Pinzi L, Rastelli G (2019) Molecular docking: shifting paradigms in drug discovery. Int J Mol Sci 20(18):4331
Qamar MT, Alqahtani SM, Alamri MA, Chen L-L (2020) Structural basis of SARS-CoV-2 3CL pro and anti-COVID-19 drug discovery from medicinal plants. J Pharm Anal 10(4):313–319
Quintão NL, da Silva GF, Antonialli CS, Rocha LW, Cechinel Filho V, Cicció JF (2010) Chemical composition and evaluation of the anti-hypernociceptive effect of the essential oil extracted from the leaves of Ugni myricoides on inflammatory and neuropathic models of pain in mice. Planta Med 76(13):1411–1418
Rana VS, Verdeguer M, Blazquez MA (2012) Chemical composition of the essential oil of Zingiber Zerumbet var. Darcyi. Nat Prod Commun 7(10):1934578X1200701031
Rasoanaivo P, Randriana RF, Maggi F, Nicoletti M, Quassinti L, Bramucci M, Lupidi G, Petrelli D, Vitali L. A, Papa F, Vittori S (2013) Chemical composition and biological activities of the essential oil of Athanasia browniiHochr. (Asteraceae) Endemic to Madagascar. Chem Biodivers 10(10):1876–1886
Ratia K, Kilianski A, Baez-Santos YM, Baker SC, Mesecar A (2014) Structural basis for the ubiquitin-linkage specificity and deISGylating activity of SARS-CoV papain-like protease. PLoS Pathog 10(5):e1004113
Ratia K, Saikatendu KS, Santarsiero BD, Barretto N, Baker SC, Stevens RC, Mesecar AD (2006) Severe acute respiratory syndrome coronavirus papain-like protease: structure of a viral deubiquitinating enzyme. Proc Natl Acad Sci USA 103(15):5717–5722
Raut JS, Karuppayil SM (2014) A status review on the medicinal properties of essential oils. Ind Crop Prod 62:250–264
Reichling J (2018) Plant-microbe interactions and secondary metabolites with antibacterial, antifungal and antiviral properties. Annual Plant Rev 2018:214–347
Rungqu P, Oyedeji O, Nkeh-Chungag B, Songca S, Oluwafemi O, Oyedeji A (2016) Anti-inflammatory activity of the essential oils of Cymbopogon validus (Stapf) Stapf ex Burtt Davy from Eastern Cape, South Africa. Asian Pac J Trop Med 9(5):426–431
Sadlish H, Galicia-Vazquez G, Paris CG, Aust T, Bhullar B, Chang L, Helliwell SB, Hoepfner D, Knapp B, Riedl R, Roggo S, Schuierer S, Studer C, Porco JA Jr, Pelletier J, Movva NR (2013) Evidence for a functionally relevant rocaglamide binding site on the eIF4A-RNA complex. ACS Chem Biol 8:1519–1527
Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB (2020) Pharmacologic treatments for coronavirus disease 2019 (COVID-19) a review. JAMA 323(18):1824–1836
Schrauf C, Kirchberger S, Majdic O, Seyerl M, Zlabinger G, Stuhlmeier KM, Sachet M, Seipelt J, Stöckl J (2009) The ssRNA genome of human rhinovirus induces a Type I IFN response but fails to induce maturation in human monocyte-derived dendritic cells. J Immunol 183(7):4440–4448
Schnitzler P (2019) Essential oils for the treatment of herpes simplex virus infections. Chemotherapy 64(1):1–7
Seibert JB, Viegas JSR, Almeida TC, Amparo TR, Rodrigues IV, Lanza JS, Frézard FJG, Soares RDOA, Teixeira LFM, Souza GHB, Vieira PMA, Barichello JM, Santos ODH (2019) Nanostructured systems improve the antimicrobial potential of the essential oil from Cymbopogon densiflorus leaves. J Nat Prod 82(12):3208–3220
Serafin MB, Bottega A, Foletto VS, Rosa TF, Hörner A, Hörner R (2020) Drug repositioning is an alternative for the treatment of coronavirus COVID-19. Int J Antimicrob Agents 55(6):105969
Sharma AD, Kaur I (2020) Eucalyptol (1,8 cineole) from eucalyptus essential oil a potential inhibitor of COVID 19 corona virus infection by molecular docking studies. Europe PMC. https://doi.org/10.20944/preprints202003.0455.v1
Sharma P, Shah GC (2015) Composition and antioxidant activity of Senecio nudicaulis Wall. ex DC. (Asteraceae): A medicinal plant growing wild in Himachal Pradesh, India. Nat Prod Res 29(9):883–886
Shayeganmehr A, Vasfi Marandi M, Karimi V, Barin A, Ghalyanchilangeroudi A (2018) Zataria multiflora essential oil reduces replication rate of avian influenza virus (H9N2 subtype) in challenged broiler chicks. Br Poult Sci 59(4):389–395
Silva EAJ, Estevam EBB, Silva TS, Nicolella HD, Furtado RA, Alves CCF, Souchie EL, Martins CHG, Tavares DC, Barbosa LCA, Miranda MLD (2018) Antibacterial and antiproliferative activities of the fresh leaf essential oil of Psidium guajava L. (Myrtaceae). Braz J Biol 79(4):697–702
Silva AC, Diodato JS, Castro JW, Matias EFF, Silva LE, do Amaral W, Maia BHLNS, Ferriani AP, Souza AK, Quintans-Júnior LJ, Coutinho HDM (2019) Effect of the essential oils from Piper sp. and blue led lights in the enhancement of the antibiotic activity of drugs against mdr bacterial strains. J Photochem Photobiol B 199:111604
Silva JKR, Figueiredo PLB, Byler KG, Setzer WN (2020) Essential oils as antiviral agents. Potential of essential oils to treat SARS-CoV-2 infection: an in-silico investigation. Int J Mol Sci 21(10):3426
Singh B, Ryan H, Kredo T, Chaplin M, Fletcher T (2020) Is chloroquine or hydroxychloroquine useful in treating people with COVID-19, or in preventing infection in people who have been exposed to the virus? Cochrane Dat Syst Rev 4:CD013587
Soares MG, Batista-Pereira LG, Fernandes JB, Corrêa AG, Da Silva MFGF, Vieira PC, Rodrigues-Filho E, Ohashi OS (2003) Electrophysiological responses of female and male Hypsipyla grandella (Zeller) to Swietenia macrophylla essential oils. J Chem Ecol 29(9):2143–2151
Steffensen KR, Gustafsson J (2004) Putative metabolic effects of the liver X receptor (LXR). Diabetes 53(Suppl 1):S36-42
Tellez MR, Dayan FE, Schrader KK, Wedge DE, Duke SO (2000) Composition and some biological activities of the essential oil of Callicarpa americana (L.). J Agric Food Chem 48(7):3008–3012
Usai M, Marchetti M, Culeddu N, Mulas M (2018) Chemical composition of myrtle (Myrtus communis L.) berries essential oils as observed in a collection of genotypes. Molecules 23(10):2502
Valdivieso-Ugarte M, Gomez-Llorente C, Plaza-Díaz J, Gil Á (2019) Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: a systematic review. Nutrients 11(11):2786
Valli M, Bolzani VS (2019) Natural products: perspectives and challenges for use of Brazilian plant species in the bioeconomy. Acad Bras Ciênc 91(3):e20190208
Vankadari N (2020) Arbidol: a potential antiviral drug for the treatment of SARS-CoV-2 by blocking trimerization of the spike glycoprotein. Int J Antimicrob Agents 56(2):105998
Velthuis AJW (2014) Common and unique features of viral RNA-dependent polymerases. Cell Mol Life Sci 71:4403–4420
Vukovic N, Milosevic T, Sukdolak S, Solujic S (2007) Antimicrobial activities of essential oil and methanol extract of Teucrium montanum. Evid Bas Complement Altern Med 4(S1):17–20
Walls AC, Park Y, Tortorici MA, Wall A, McGuire AT, Veesler D (2020) Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181(2):281–292
Wang S, Li Y, Wang J, Chen L, Zhang L, Yu H, Hou T (2012) ADMET evaluation in drug discovery. 12. Development of binary classification models for prediction of hERG potassium channel blockage. Mol Pharmaceutics 9:996–1010
Wang CF, Yang K, You CX, Zhang WJ, Guo SS, Geng ZF, Du SS, Wang YY (2015) Chemical composition and insecticidal activity of essential oils from Zanthoxylum dissitum leaves and roots against three species of storage pests. Molecules 20(5):7990–7999
Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G (2020) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30:269–271
Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C, Abiona O, Graham BS, McLellan JS (2020) Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367(6483):1260–1263
Wu Y, Guo SS, Huang DY, Wang CF, Wei JY, Li ZH, Sun JS, Bai JF, Wang PJ, Du SS (2017) Contact and repellant activities of zerumbone and its analogues from the Essential Oil of Zingiber zerumbet (L.) Smith against Lasioderma serricorne. J Oleo Sci 66(4):399–405
Wu C, Liu Y, Yang Y, Zhang P, Zhong W, Wang Y, Wang Q, Xu Y, Li M, Li X, Zheng M, Chen L, Li H (2020) Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sinica B 10(5):766–788
Xie L, Xie L, Bourne PE (2011) Structure-based systems biology for analyzing off-target binding. Curr Opin Struct Biol 21(2):189–199
Yamamoto N, Yang R, Yoshinaka Y, Amari S, Nakano T, Cinatl J, Rabenau H, Doerr HW, Hunsmann G, Otaka A, Tamamura H, Fujii N, Yamamoto N (2004) HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus. Biochem Biophys Res Commun 318(3):719–725
Yamamoto N, Matsuyama S, Hoshino T, Yamamoto N (2020) Nelfinavir inhibits replication of severe acute respiratory syndrome coronavirus 2 in vitro. ChemRxiv. https://doi.org/10.1101/2020.04.06.026476
Yamaya M, Nishimura H, Deng X, Sugawara M, Watanabe O, Nomura K, Shimotai Y, Momma H, Ichinose M, Kawase T (2020) Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respir Investig 58(3):155–168
Zhang L, Lin D, Sun X, Curth U, Drosten C, Sauerhering L, Becker S, Rox K, Hilgenfeld R (2020) Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide. Science 368(6489):409–412
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Phil D, Tan WN (2020a) A novel coronavirus from patients with pneumonia in China. Engl J Med 382(8):727–733
Zhu Z, Lu Z, Xu T, Chen C, Yang G, Zha T, Lu J, Xue Y (2020b) Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J Infect. https://doi.org/10.1016/j.jinf.2020.03.060
Zou L, Dai L, Zhang X, Zhang Z, Zhang Z (2020c) Hydroxychloroquine and chloroquine: a potential and controversial treatment for COVID-19. Arch Pharm Res 43:765–772
Acknowledgements
Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Universidade Federal de Ouro Preto.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Amparo, T.R., Seibert, J.B., Silveira, B.M. et al. Brazilian essential oils as source for the discovery of new anti-COVID-19 drug: a review guided by in silico study. Phytochem Rev 20, 1013–1032 (2021). https://doi.org/10.1007/s11101-021-09754-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-021-09754-4