Abstract
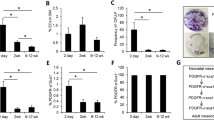
Mesenchymal stem cells (MSCs) are known to have many notable features, especially their multiple differentiation ability and immunoregulatory capacity. MSCs are important stem cells in the bone marrow (BM), and their characteristics are affected by the BM microenvironment. However, effects of the BM microenvironment on the properties of MSCs are not well understood. In this study, we found that BM from aged mice decreased MSC colony formation. Flow cytometry data showed that the proportion of B220+ cells in BM from aged mice was significantly lower than that in BM from young mice, while the proportion of CD11b+, CD3+, Gr-1+, or F4/80+ cells are on the contrary. CD11b+, B220+, and Ter119+ cells from aged mice were not the subsets that decreased MSC colony formation. We further demonstrated that both BM from aged mice and young mice exhibited similar effects on the proliferation of murine MSC cell line C3H10T1/2. However, when cocultured with BM from aged mice, C3H10T1/2 showed slower migration ability. In addition, we found that phosphorylation of JNK (c-Jun N-terminal kinases) in C3H10T1/2 cocultured with BM from aged mice was lower than that in C3H10T1/2 cocultured with BM from young mice. Collectively, our data revealed that BM from aged mice could decrease the migration of MSCs from their niche through regulating the JNK pathway.






Similar content being viewed by others
References
Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS (2003) Effect of stromal cell derived factor-1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet 362:697–703
Bruder SP, Jaiswal N, Haynesworth SE (1997) Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biol Chem 64:278–294
Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT (2003) Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425:841–846
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Choop M (2001) Therapeutic benefit of intravenousadministration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 32:1005–1011
Chen FM, Wu LA, Zhang M, Zhang R, Sun HH (2011) Homing of endogenous stem/progenitor cells for in situ tissue regeneration: promises, strategies, and translational perspectives. Biomaterials 32:3189–3209
Chen LZ, Yin SM, Zhang XL, Chen JY, Wei BX, Zhan Y, Yu W, Wu JM, Qu J, Guo ZK (2012) Protective effects of human bone marrow mesenchymalstemcells on hematopoietic organs of irradiated mice. Zhongguo Shi Yan Xue Ye Xue Za Zhi 20:1436–1441
Chishti MA, Kaya N, Binbakheet AB, Al-Mohanna F, Goyns MH, Colak D (2013) Induction of cell proliferation in old rat liver can reset certain gene expression levels characteristic of old liver to those associated with young liver. Age (Dordr) 35:719–732
D’Agostino B, Sullo N, Siniscalco D, De Angelis A, Rossi F (2010) Mesenchymal stem cell therapy for the treatment of chronic obstructive pulmonary disease. Expert Opin Biol Ther 10:681–687
Dazzi F, Ramasamy R, Glennie S, Jones SP, Roberts I (2006) The role of mesenchymal stem cells in haemopoiesis. Blood Rev 20:161–171
de Haan G, Nijhof W, Van Zant G (1997) Mouse strain-dependent changes in frequency and proliferation of hematopoietic stem cells during aging: correlation between lifespan and cycling activity. Blood 89:1543–1550
Devine SM, Bartholomew AM, Mahmud N, Nelson M, Patil S, Hardy W, Sturgeon C, Hewett T, Chung T, Stock W, Sher D, Weissman S, Ferrer K, Mosca J, Deans R, Moseley A, Hoffman R (2001) Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol 29:244–255
Donnini A, Re F, Orlando F, Provinciali M (2007) Intrinsic and microenvironmental defects are involved in the age-related changes of Lin-ckit + hematopoietic progenitor cells. Rejuvenation Res 10:459–472
Gao H, Priebe W, Glod J, Banerjee D (2009) Activation of signal transducers and activators of transcription 3 and focal adhesion kinase by stromal cell-derived factor 1 is required for migration of human mesenchymal stem cells in response to tumor cellconditioned medium. Stem Cells 27:857–865
Li F, Jin F, Freitas A, Szabo P, Weksler ME (2001) Impaired regeneration of the peripheral B cell repertoire from bone marrow following lymphopenia in old mice. Eur J Immunol 31:500–505
Li ZY, Wang CQ, Lu G (2011) Effects of bone marrow mesenchymal stem cells on hematopoietic recovery and acute graft-versus-host disease in murine allogeneic umbilical cord blood transplantation model. Zhonghua Xue Ye Xue Za Zhi 32:786–789
Loebinger MR, Eddaoudi A, Davies D, Janes SM (2009) Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res 69:4134–4142
Mazzini L, Ferrero I, Luparello V, Rustichelli D, Gunetti M, Mareschi K, Testa L, Stecco A, Tarletti R, Miglioretti M, Fava E, Nasuelli N, Cisari C, Massara M, Vercelli R, Oggioni GD, Carriero A, Cantello R, Monaco F, Fagioli F (2010) Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: a Phase I clinical trial. Exp Neurol 223:229–237
Min H, Montecino-Rodriguez E, Dorshkind K (2006) Effects of aging on the common lymphoid progenitor to pro-Bcell transition. J Immunol 176:1007–1012
Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL (1996) The aging of hematopoietic stem cells. Nat Med 2:1011–1016
Muslin AJ (2008) MAPK signalling in cardiovascular health and disease: molecular mechanisms and therapeutic targets. Clin Sci (Lond) 115:203–218
Prockop DJ (1997) Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276:71–74
Shao HW, Xu QY, Wu QL, Ma Q, Salqueiro L, Wang J, Eton D, Webster KA, Yu H (2011) Defective CXCR4 expression in aged bone marrow cells impairs vascular regeneration. J Cell Mol Med 15:2046–2056
Sudo K, Ema H, Morita Y, Nakauchi H (2000) Age-associated characteristics of murine hematopoietic stem cells. J Exp Med 192:1273–1280
Veronesi F, Pagani S, Della Bella E, Giavaresi G, Fini M (2014) Estrogen deficiency does not decrease the in vitro osteogenic potential of rat adipose-derived mesenchymal stem cells. Age 36:1225–1238
Wang MC, Bohmann D, Jasper H (2005) JNK extends life span and limits growth by antagonizing cellular and organism wide responses to insulin signaling. Cell 121:115–125
Waterstrat A, Van Zant G (2009) Effects of aging on hematopoietic stem and progenitor cells. Curr Opin Immunol 21:408–413
Yang YM, Li H, Zhang L, Dang RJ, Li P, Wang XY, Zhu H, Guo XM, Zhang Y, Liu YL, Mao N, Jiang XX, Wen N (2013) A new method for isolating and culturing mouse bone marrow mesenchymal stem cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 21:1563–1567
Yuan L, Sakamoto N, Song G, Sato M (2013) Low-level shear stress induces human mesenchymal stem cell migration through the SDF-1/CXCR4 axis via MAPK signaling pathways. Stem Cell Dev 22:2384–2393
Zediak VP, Maillard I, Bhandoola A (2007) Multiple prethymic defects underlie age-related loss of T progenitor competence. Blood 110:1161–1167
Zhu X, Gui J, Dohkan J, Cheng L, Barnes PF, Su DM (2007) Lymphohematopoietic progenitors do not have a synchronized defect with age-related thymic involution. Aging Cell 6:663–672
Acknowledgments
The authors wish to thank Lindsey Jones (Keck School of Medicine, University of Southern California) for her critical reading of the paper. This study was supported by grants from the National Natural Science Foundation of China (81271936), the Chinese National Key Program on Basic Research (2010CB529904).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yan-Mei Yang and Ping Li contributed equally to this work.
About this article
Cite this article
Yang, YM., Li, P., Cui, DC. et al. Effect of aged bone marrow microenvironment on mesenchymal stem cell migration. AGE 37, 16 (2015). https://doi.org/10.1007/s11357-014-9743-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11357-014-9743-z