Abstract
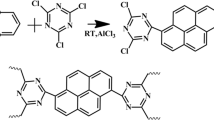
We exploited a unique porous structure of the nano-covalent triazine polymer (NCTP) containing aggregation-induced emission (AIE) group to achieve controlled release and drug tracking in tumor acidic microenvironment. NCTP was synthesized by the Friedel-Crafts alkylation and the McMurry coupling reaction. It not only had strong doxorubicin (DOX)-loading capacity due to its high specific surface area and large pore volume, but also showed the significant cumulative drug release as a result of the pH response of triazine polymers. NCTP was induced luminescence after mass accumulation near tumor cells. Besides, it had excellent biocompatibility and obvious antineoplastic toxicity. The results demonstrate that NCTP as a utility-type drug carrier provides a new route for designing the multi-functional drug delivery platform.
Access this article
We’re sorry, something doesn't seem to be working properly.
Please try refreshing the page. If that doesn't work, please contact support so we can address the problem.
Similar content being viewed by others
References
Weingart S N, Zhang L, Sweeney M, et al. Chemotherapy medication errors. Lancet Oncology, 2018, 19(4): e191–e199
Liu P, Zhang R, Pei M. Design of pH/reduction dual-responsive nanoparticles as drug delivery system for DOX: Modulating controlled release behavior with bimodal drug-loading. Colloids and Surfaces B: Biointerfaces, 2017, 160(27): 455–461
Tap W D, Wagner A J, Schöffski P, et al. Effect of doxorubicin plus olaratumab vs doxorubicin plus placebo on survival in patients with advanced soft tissue sarcomas: The announce randomized clinical trial. JAMA: Journal of the American Medical Association, 2020, 323(13): 1266–1276
Krishnan V, Rajasekaran A K. Clinical nanomedicine: a solution to the chemotherapy conundrum in pediatric leukemia therapy. Clinical Pharmacology and Therapeutics, 2014, 95(2): 168–178
Xiong H, Wang Z, Wang C, et al. Transforming complexity to simplicity: protein-like nanotransformer for improving tumor drug delivery programmatically. Nano Letters, 2020, 20(3): 1781–1790
Peer D, Karp J M, Hong S, et al. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnology, 2007, 2(12): 751–760
Wilhelm S, Tavares A J, Dai Q, et al. Analysis of nanoparticle delivery to tumours. Nature Reviews: Materials, 2016, 1(5): 16014
Chai Z, Ran D, Lu L, et al. Ligand-modified cell membrane enables the targeted delivery of drug nanocrystals to glioma. ACS Nano, 2019, 13(5): 5591–5601
Ding B, Shao S, Yu C, et al. Large-pore mesoporous-silica-coated upconversion nanoparticles as multifunctional immunoadjuvants with ultrahigh photosensitizer and antigen loading efficiency for improved cancer photodynamic immunotherapy. Advanced Materials, 2018, 30(52): 1802479
Seynhaeve A L B, Amin M, Haemmerich D, et al. Hyperthermia and smart drug delivery systems for solid tumor therapy. Advanced Drug Delivery Reviews, 2020, 32: 89–95
Su C, Liu Y, Li R, et al. Absorption, distribution, metabolism and excretion of the biomaterials used in nanocarrier drug delivery systems. Advanced Drug Delivery Reviews, 2019, 143: 97–114
Wang H, Jiang D, Huang D, et al. Covalent triazine frameworks for carbon dioxide capture. Journal of Materials Chemistry A: Materials for Energy and Sustainability, 2019, 7(40): 22848–22870
Yang Y, Niu H, Xu L, et al. Triazine functionalized fully conjugated covalent organic framework for efficient photocatalysis. Applied Catalysis B: Environmental, 2020, 269: 118799
Ji D, Li T, Fuchs H. Patterning and applications of nanoporous structures in organic electronics. Nano Today, 2020, 31: 100843
Rengaraj A, Puthiaraj P, Haldorai Y, et al. Porous covalent triazine polymer as a potential nanocargo for cancer therapy and imaging. ACS Applied Materials & Interfaces, 2016, 8(14): 8947–8955
Lu B, Li L, Wei L, et al. Synthesis and thermo-responsive self-assembly behavior of amphiphilic copolymer β-CD-(PCL-P (MEO2MA-co-PEGMA))21 for the controlled intracellular delivery of doxorubicin. RSC Advances, 2016, 6(56): 50993–51004
Wei L, Lu B, Li L, et al. One-step synthesis and self-assembly behavior of thermo-responsive star-shaped β-cyclodextrin-(P (MEO2MA-co-PEGMA))21 copolymers. Frontiers of Materials Science, 2017, 11(3): 223–232
Peng X, Wei L, Jing X, et al. Stimuli-responsive nano-polymer composite materials based on the triazine skeleton structure used in drug delivery. JOM, 2019, 71(1): 308–314
Wei L, Lu B, Cui L, et al. Folate-conjugated pH-responsive nanocarrier designed for active tumor targeting and controlled release of doxorubicin. Frontiers of Materials Science, 2017, 11 (4): 328–343
Lu B B, Wei L L, Meng G H, et al. Synthesis of self-assemble pH-responsive cyclodextrin block copolymer for sustained anticancer drug delivery. Chinese Journal of Polymer Science, 2017, 35(8): 924–938
Murugesan S, Scheibel T. Copolymer/clay nanocomposites for biomedical applications. Advanced Functional Materials, 2020, 30 (17): 1908101
Niu G, Zhang R, Shi X, et al. AIE luminogens as fluorescent bioprobes. Trends in Analytical Chemistry, 2020, 123: 115769
Ding D, Mao D, Li K, et al. Precise and long-term tracking of adipose-derived stem cells and their regenerative capacity via superb bright and stable organic nanodots. ACS Nano, 2014, 8 (12): 12620–12631
Wang Y, Chen M, Alifu N, et al. Aggregation-induced emission luminogen with deep-red emission for through-skull three-photon fluorescence imaging of mouse. ACS Nano, 2017, 11(10): 10452–10461
Gui C, Zhao E, Kwok R T K, et al. AIE-active theranostic system: Selective staining and killing of cancer cells. Chemical Science, 2017, 8(3): 1822–1830
Zhang R, Niu G, Lu Q, et al. Cancer cell discrimination and dynamic viability monitoring through wash-free bioimaging using AIEgens. Chemical Science, 2020, 11(29): 7676–7684
Zhang P, Zhao Z, Li C, et al. Aptamer-decorated self-assembled aggregation-induced emission organic dots for cancer cell targeting and imaging. Analytical Chemistry, 2018, 90(2): 1063–1067
Zhang H, Zhao Z, Turley AT, et al. Aggregate science: From structures to properties. Advanced Materials, 2020, 32(36): 2001457
Zhang J, Liang M, Wang X, et al. Visualizing peroxynitrite fluxes in myocardial cells using a new fluorescent probe reveals the protective effect ofestrogen. Chemical Communications, 2019, 55 (47): 6719–6722
Rodríguez-Nuévalos S, Parra M, Ceballos S, et al. A nitric oxide induced “click” reaction to trigger the aggregation induced emission (AIE) phenomena of a tetraphenyl ethylene derivative: A new fluorescent probe for NO. Journal of Photochemistry and Photobiology A: Chemistry, 2020, 388: 112132
Chen X, Gao H, Deng Y, et al. Supramolecular aggregation-induced emission nanodots with programmed tumor microenvironment responsiveness for image-guided orthotopic pancreatic cancer therapy. ACS Nano, 2020, 14(4): 5121–5134
Kim Y H, Jeong H C, Kim S H, et al. High-purity-blue and high-efficiency electroluminescent devices based on anthracene. Advanced Functional Materials, 2005, 15(11): 1799–1805
Konidena R K, Lee K H, Lee J Y. Two-channel emission controlled by a conjugation valve for the color switching of thermally activated delayed fluorescence emission. Journal of Materials Chemistry C: Materials for Optical and Electronic Devices, 2019, 7(32): 9908–9916
Rengaraj A, Puthiaraj P, Haldorai Y, et al. Porous covalent triazine polymer as a potential nanocargo for cancer therapy and imaging. ACS Applied Materials & Interfaces, 2016, 8(14): 8947–8955
Lee H J, Lee H G, Kwon Y B, et al. The role of lactose carrier on the powder behavior and aerodynamic performance of bosentan microparticles for dry powder inhalation. European Journal of Pharmaceutical Sciences, 2018, 117: 279–289
Guo B, Wu M, Shi Q, et al. All-in-one molecular aggregation-induced emission theranostics: fluorescence image guided and mitochondria targeted chemo- and photodynamic cancer cell ablation. Chemistry of Materials, 2020, 32(11): 4681–4691
Wang S, Chen H, Liu J, et al. NIR-II light activated photosensitizer with aggregation-induced emission for precise and efficient two-photon photodynamic cancer cell ablation. Advanced Functional Materials, 2020, 30(30): 2002546
Mei J, Leung N L C, Kwok R T K, et al. Aggregation-induced emission: together we shine, united we soar! Chemical Reviews, 2015, 115(21): 11718–11940
Wang S, Hu F, Pan Y, et al. Bright AIEgen-protein hybrid nanocomposite for deep and high-resolution in vivo two-photon brain imaging. Advanced Functional Materials, 2019, 29(29): 1902717
Kozai T D Y, Jaquins-Gerstl A S, Vazquez A L, et al. Dexamethasone retrodialysis attenuates microglial response to implanted probes in vivo. Biomaterials, 2016, 87: 157–169
Liu L H, Qiu W X, Zhang Y H, et al. A charge reversible self-delivery chimeric peptide with cell membrane-targeting properties for enhanced photodynamic therapy. Advanced Functional Materials, 2017, 27(25): 1700220
Acknowledgements
This work was supported by the Corps Division Development and Innovation Support Program (2017BA041), the National Natural Science Foundation of China (Grant Nos. 21866028 and 51662036), the Engineering Research Center of Materials-Oriented Chemical Engineering of Xinjiang Bintuan (2016BTRC008 and 2016BTRC005) and the Natural Science Foundation of Shihezi University (ZZZC201922A).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Disclosure of potential conflicts of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, Y., Peng, X., Jing, X. et al. Synthesis of pH-responsive triazine skeleton nano-polymer composite containing AIE group for drug delivery. Front. Mater. Sci. 15, 113–123 (2021). https://doi.org/10.1007/s11706-021-0539-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11706-021-0539-7