Abstract
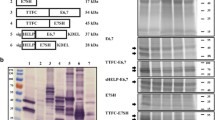
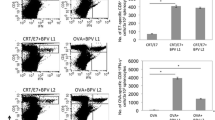
Ubiquitin–proteasome system plays an essential role in the immune response due to its involvement in the antigen generation and presentation to CD8+ T cells. Hereby, ubiquitin fused to antigens has been explored as an immunotherapeutic strategy that requires the activation of cytotoxic T lymphocytes. Here we propose to apply this ubiquitin fusion approach to a recombinant vaccine against human papillomavirus 16-infected cells. E6E7 multi-epitope antigen was fused genetically at its N- or C-terminal end to ubiquitin and expressed in Escherichia coli as inclusion bodies. The antigens were solubilized using urea and purified by nickel affinity chromatography in denatured condition. Fusion of ubiquitin to E6E7 resulted in marked polyubiquitination in vitro mainly when fused to the E6E7 N-terminal. When tested in a therapeutic scenario, the fusion of ubiquitin to E6E7 reinforced the anti-tumor protection and increased the E6/E7-specific cellular immune responses. Present results encourage the investigation of the adjuvant potential of the ubiquitin fusion to recombinant vaccines requiring CD8+ T cells.







Similar content being viewed by others
Abbreviations
- Ub:
-
Ubiquitin
- MHC:
-
Major histocompatibility complex
- APC:
-
Antigen-presenting cells
- LB:
-
Luria–Bertani
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- PBS:
-
Phosphate-buffered saline
- DTT:
-
1,4-Dithiothreitol
References
Hershko, A., & Ciechanover, A. (1998). The ubiquitin system. Annual Review of Biochemistry, 67, 425–479.
Coux, O., Tanaka, K., & Goldberg, A. L. (1996). Structure and functions of the 20S and 26S proteasomes. Annual Review of Biochemistry, 65, 801–847.
Hochstrasser, M. (1996). Ubiquitin-dependent protein degradation. Annual Review of Genetics, 30, 405–439.
Aldarouish, M., Wang, H., Zhou, M., Hu, H. M., & Wang, L. X. (2015). Ubiquitinated proteins enriched from tumor cells by a ubiquitin binding protein Vx3(A7) as a potent cancer vaccine. Journal of Experimental & Clinical Cancer Research, 34, 34.
Chou, B., Hiromatsu, K., Okano, S., Ishii, K., Duan, X., Sakai, T., et al. (2012). Antiangiogenic tumor therapy by DNA vaccine inducing aquaporin-1-specific CTL based on ubiquitin–proteasome system in mice. Journal of Immunology, 189, 1618–1626.
Eslami, N. S., Shokrgozar, M. A., Mousavi, A., Azadmanesh, K., Nomani, A., Apostolopoulos, V., et al. (2012). Simultaneous immunisation with a Wilms’ tumour 1 epitope and its ubiquitin fusions results in enhanced cell mediated immunity and tumour rejection in C57BL/6 mice. Molecular Immunology, 51, 325–331.
Velders, M. P., Weijzen, S., Eiben, G. L., Elmishad, A. G., Kloetzel, P. M., Higgins, T., et al. (2001). Defined flanking spacers and enhanced proteolysis is essential for eradication of established tumors by an epitope string DNA vaccine. Journal of Immunology, 166, 5366–5373.
Yin, H., Zhao, L., Wang, T., Zhou, H., He, S., & Cong, H. (2015). A Toxoplasma gondii vaccine encoding multistage antigens in conjunction with ubiquitin confers protective immunity to BALB/c mice against parasite infection. Parasites & Vectors, 8, 498.
Ciechanover, A. (2005). N-terminal ubiquitination. Methods in Molecular Biology, 301, 255–270.
Ciechanover, A., & Ben-Saadon, R. (2004). N-terminal ubiquitination: more protein substrates join in. Trends in Cell Biology, 14, 103–106.
Aviel, S., Winberg, G., Massucci, M., & Ciechanover, A. (2000). Degradation of the epstein-barr virus latent membrane protein 1 (LMP1) by the ubiquitin–proteasome pathway. Targeting via ubiquitination of the N-terminal residue. The Journal of Biological Chemistry, 275, 23491–23499.
Bloom, J., Amador, V., Bartolini, F., DeMartino, G., & Pagano, M. (2003). Proteasome-mediated degradation of p21 via N-terminal ubiquitinylation. Cell, 115, 71–82.
Coulombe, P., Rodier, G., Bonneil, E., Thibault, P., & Meloche, S. (2004). N-Terminal ubiquitination of extracellular signal-regulated kinase 3 and p21 directs their degradation by the proteasome. Molecular and Cellular Biology, 24, 6140–6150.
Fajerman, I., Schwartz, A. L., & Ciechanover, A. (2004). Degradation of the Id2 developmental regulator: targeting via N-terminal ubiquitination. Biochemical and Biophysical Research Communications, 314, 505–512.
Ikeda, M., Ikeda, A., & Longnecker, R. (2002). Lysine-independent ubiquitination of Epstein-Barr virus LMP2A. Virology, 300, 153–159.
Reinstein, E., Scheffner, M., Oren, M., Ciechanover, A., & Schwartz, A. (2000). Degradation of the E7 human papillomavirus oncoprotein by the ubiquitin–proteasome system: targeting via ubiquitination of the N-terminal residue. Oncogene, 19, 5944–5950.
Breitschopf, K., Bengal, E., Ziv, T., Admon, A., & Ciechanover, A. (1998). A novel site for ubiquitination: the N-terminal residue, and not internal lysines of MyoD, is essential for conjugation and degradation of the protein. EMBO Journal, 17, 5964–5973.
Papagatsias, T., Athanasopoulos, T., Meiser, A., Benlahrech, A., Li, F., Self, S., et al. (2009). Using ubiquitin fusion to augment CD8+ T cell immune responses against HIV-1 antigens. Retrovirology. doi:10.1186/1742-4690-6-S3-P303.
Rodriguez, F., An, L. L., Harkins, S., Zhang, J., Yokoyama, M., Widera, G., et al. (1998). DNA immunization with minigenes: low frequency of memory cytotoxic T lymphocytes and inefficient antiviral protection are rectified by ubiquitination. Journal of Virology, 72, 5174–5181.
Setz, C., Friedrich, M., Hahn, S., Dorrie, J., Schaft, N., Schuler, G., et al. (2013). Just one position-independent lysine residue can direct MelanA into proteasomal degradation following N-terminal fusion of ubiquitin. PLoS ONE, 8, e55567.
Zhang, M., Ishii, K., Hisaeda, H., Murata, S., Chiba, T., Tanaka, K., et al. (2004). Ubiquitin-fusion degradation pathway plays an indispensable role in naked DNA vaccination with a chimeric gene encoding a syngeneic cytotoxic T lymphocyte epitope of melanocyte and green fluorescent protein. Immunology, 112, 567–574.
Andersson, H. A., & Barry, M. A. (2004). Maximizing antigen targeting to the proteasome for gene-based vaccines. Molecular Therapy, 10, 432–446.
Johnson, E. S., Ma, P. C., Ota, I. M., & Varshavsky, A. (1995). A proteolytic pathway that recognizes ubiquitin as a degradation signal. Journal of Biological Chemistry, 270, 17442–17456.
Chen, J. H., Yu, Y. S., Liu, H. H., Chen, X. H., Xi, M., Zang, G. Q., et al. (2011). Ubiquitin conjugation of hepatitis B virus core antigen DNA vaccine leads to enhanced cell-mediated immune response in BALB/c mice. Hepatitis Monthly, 11, 620–628.
Duan, X., Hisaeda, H., Shen, J., Tu, L., Imai, T., Chou, B., et al. (2006). The ubiquitin–proteasome system plays essential roles in presenting an 8-mer CTL epitope expressed in APC to corresponding CD8+ T cells. International Immunology, 18, 679–687.
van der Burg, S. H., & Melief, C. J. (2011). Therapeutic vaccination against human papilloma virus induced malignancies. Current Opinion in Immunology, 23, 252–257.
Hellner, K., & Munger, K. (2011). Human papillomaviruses as therapeutic targets in human cancer. Journal of Clinical Oncology, 29, 1785–1794.
Leachman, S. A., Shylankevich, M., Slade, M. D., Levine, D., Sundaram, R. K., Xiao, W., et al. (2002). Ubiquitin-fused and/or multiple early genes from cottontail rabbit papillomavirus as DNA vaccines. Journal of Virology, 76, 7616–7624.
Park, M. J., Kim, E. K., Han, J. Y., Cho, H. W., Sohn, H. J., Kim, S. Y., et al. (2010). Fusion of the Human Cytomegalovirus pp65 antigen with both ubiquitin and ornithine decarboxylase additively enhances antigen presentation to CD8(+) T cells in human dendritic cells. Human Gene Therapy, 21, 957–967.
Sasawatari, S., Tadaki, T., Isogai, M., Takahara, M., Nieda, M., & Kakimi, K. (2006). Efficient priming and expansion of antigen-specific CD8+ T cells by a novel cell-based artificial APC. Immunology and Cell Biology, 84, 512–521.
de Oliveira, L. M., Morale, M. G., Chaves, A. A., Cavalher, A. M., Lopes, A. S., Diniz Mde, O., et al. (2015). Design, immune responses and anti-tumor potential of an HPV16 E6E7 multi-epitope vaccine. PLoS ONE, 10, e0138686.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Ramos, C. R., Abreu, P. A., Nascimento, A. L., & Ho, P. L. (2004). A high-copy T7 Escherichia coli expression vector for the production of recombinant proteins with a minimal N-terminal His-tagged fusion peptide. Brazilian Journal of Medical and Biological Research, 37, 1103–1109.
Froger, A., & Hall, J. E. (2007). Transformation of plasmid DNA into E. coli using the heat shock method. Journal of Visualized Experiments, 1, 253.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
Zhang, M., Obata, C., Hisaeda, H., Ishii, K., Murata, S., Chiba, T., et al. (2005). A novel DNA vaccine based on ubiquitin–proteasome pathway targeting `self’-antigens expressed in melanoma/melanocyte. Gene Therapy, 12, 1049–1057.
Brandsma, J. L., Shlyankevich, M., Zelterman, D., & Su, Y. (2007). Therapeutic vaccination of rabbits with a ubiquitin-fused papillomavirus E1, E2, E6 and E7 DNA vaccine. Vaccine, 25, 6158–6163.
Xiang, R., Lode, H. N., Chao, T. H., Ruehlmann, J. M., Dolman, C. S., Rodriguez, F., et al. (2000). An autologous oral DNA vaccine protects against murine melanoma. Proceedings of the National Academy of Sciences of the United States of America, 97, 5492–5497.
Yi, T., Sun, S., Huang, Y., & Chen, Y. (2015). Prokaryotic expression and mechanism of action of alpha-helical antimicrobial peptide A20L using fusion tags. BMC Biotechnology, 15, 69.
Lowe, A. J., Bardliving, C. L., Huang, C. J., Teixeira, L. M., Damasceno, L. M., Anderson, K. A., et al. (2011). Expression and purification of cGMP grade NY-ESO-1 for clinical trials. Biotechnology Progress, 27, 435–441.
Qian, S. B., Ott, D. E., Schubert, U., Bennink, J. R., & Yewdell, J. W. (2002). Fusion proteins with COOH-terminal ubiquitin are stable and maintain dual functionality in vivo. Journal of Biological Chemistry, 277, 38818–38826.
Lee, J. C., Wang, G. X., Schickling, O., & Peter, M. E. (2005). Fusing DEDD with ubiquitin changes its intracellular localization and apoptotic potential. Apoptosis, 10, 1483–1495.
Groothuis, T. A., & Neefjes, J. (2005). The many roads to cross-presentation. The Journal of Experimental Medicine, 202, 1313–1318.
Amigorena, S., & Savina, A. (2010). Intracellular mechanisms of antigen cross presentation in dendritic cells. Current Opinion in Immunology, 22, 109–117.
Joffre, O. P., Segura, E., Savina, A., & Amigorena, S. (2012). Cross-presentation by dendritic cells. Nature Reviews Immunology, 12, 557–569.
Herath, S., Benlahrech, A., Papagatsias, T., Athanasopoulos, T., Bouzeboudjen, Z., Hervouet, C., et al. (2014). Fusion of ubiquitin to HIV gag impairs human monocyte-derived dendritic cell maturation and reduces ability to induce gag T cell responses. PLoS ONE, 9, e88327.
Vidalin, O., Tanaka, E., Spengler, U., Trepo, C., & Inchauspe, G. (1999). Targeting of hepatitis C virus core protein for MHC I or MHC II presentation does not enhance induction of immune responses to DNA vaccination. DNA and Cell Biology, 18, 611–621.
Acknowledgements
The authors would like to thank Fapesp (2010/04490-4 and 2007/51698-7), CNPq (304467/2010-3 and 306992/2014-0), and Fundação Butantan for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There is no conflict of interest.
Rights and permissions
About this article
Cite this article
de Oliveira, L.M.F., Morale, M.G., Chaves, A.A.M. et al. Expression, Polyubiquitination, and Therapeutic Potential of Recombinant E6E7 from HPV16 Antigens Fused to Ubiquitin. Mol Biotechnol 59, 46–56 (2017). https://doi.org/10.1007/s12033-016-9990-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-016-9990-6