Abstract
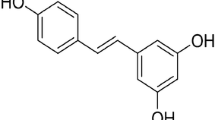
Resveratrol (3,4',5-trihydroxystilbene; C14H12O3) is a polyphenolic phytoalexin found in grapes, berries, peanuts, and wines. Resveratrol has been viewed as an antioxidant, anti-inflammatory, anti-apoptotic, and anticancer agent. Moreover, it has been reported that resveratrol modulates mitochondrial function, redox biology, and dynamics in both in vitro and in vivo experimental models. Resveratrol also attenuates mitochondrial impairment induced by certain stressors. Resveratrol upregulates, for example, mitochondria-located antioxidant enzymes, decreasing the production of reactive species by these organelles. Resveratrol also triggers mitochondrial biogenesis, ameliorating the mitochondria-related bioenergetics status in mammalian cells. In the present work, we discuss about the effects of resveratrol on brain mitochondria. Brain cells (both neuronal and glial) are susceptible to mitochondrial dysfunction due to their high demand for adenosine triphosphate (ATP). Additionally, brain cells consume oxygen (O2) at very high rates, leading to a proportionally high mitochondrial production of reactive species. Therefore, strategies focusing on the maintenance of mitochondrial function in these cell types are of pharmacological interest in the case of neurodegenerative diseases, which involve mitochondrial impairment and increased generation of reactive species, leading to neuroinflammation and cell death. The mechanism by which resveratrol protects mitochondrial function and dynamics is not completely understood, and further research would be necessary in order to investigate exactly how resveratrol affects mitochondria-related parameters. Furthermore, it is particularly important because resveratrol is able to induce cytotoxicity depending on its dosage.
Similar content being viewed by others
References
Shakibaei M, Harikumar KB, Aggarwal BB (2009) Resveratrol addiction: to die or not to die. Mol Nutr Food Res 53:115–128. doi:10.1002/mnfr.200800148
Hsieh TC, Wu JM (2010) Resveratrol: biological and pharmaceutical properties as anticancer molecule. Biofactors 36:360–369
Kalantari H, Das DK (2010) Physiological effects of resveratrol. Biofactors 36:401–406
Schnekenburger M, Dicato M, Diederich M (2014) Plant-derived epigenetic modulators for cancer treatment and prevention. Biotechnol Adv 32:1123–1132. doi:10.1016/j.biotechadv.2014.03.009
Chan S, Kantham S, Rao VM, Palanivelu MK, Pham HL, Shaw PN, McGeary RP, Ross BP (2016) Metal chelation, radical scavenging and inhibition of Aβ42 fibrillation by food constituents in relation to Alzheimer's disease. Food Chem 199:185–194. doi:10.1016/j.foodchem.2015.11.118
Blanquer-Rosselló MD, Hernández-López R, Roca P, Oliver J, Valle A (2016) Resveratrol induces mitochondrial respiration and apoptosis in SW620 colon cancer cells. Biochim Biophys Acta. doi:10.1016/j.bbagen.2016.10.009
Kopp P (1998) Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the ʻFrench paradoxʼ? Eur J Endocrinol 138:619–620
Yap S, Qin C, Woodman OL (2010) Effects of resveratrol and flavonols on cardiovascular function: physiological mechanisms. Biofactors 36:350–359
Albani D, Polito L, Signorini A, Forloni G (2010) Neuroprotective properties of resveratrol in different neurodegenerative disorders. Biofactors 36:370–376
Anandhan A, Tamilselvam K, Vijayraja D, Ashokkumar N, Rajasankar S, Manivasagam T (2010) Resveratrol attenuates oxidative stress and improves behaviour in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) challenged mice. Ann Neurosci 17:113–119. doi:10.5214/ans.0972-7531.1017304
Sun AY, Wang Q, Simonyi A, Sun GY (2010) Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol Neurobiol 41:375–383. doi:10.1007/s12035-010-8111-y
de Oliveira MR, Nabavi SF, Manayi A, Daglia M, Hajheydari Z, Nabavi SM (2016) Resveratrol and the mitochondria: from triggering the intrinsic apoptotic pathway to inducing mitochondrial biogenesis, a mechanistic view. Biochim Biophys Acta 1860:727–745. doi:10.1016/j.bbagen.2016.01.017
Ahmed T, Javed S, Javed S, Tariq A, Šamec D, Tejada S, Nabavi SF, Braidy N et al (2016) Resveratrol and Alzheimer's disease: mechanistic insights. Mol Neurobiol. doi:10.1007/s12035-016-9839-9
Simonyi A, Wang Q, Miller RL, Yusof M, Shelat PB, Sun AY, Sun GY (2005) Polyphenols in cerebral ischemia: novel targets for neuroprotection. Mol Neurobiol 31:135–147
Lin CJ, Chen TH, Yang LY, Shih CM (2014) Resveratrol protects astrocytes against traumatic brain injury through inhibiting apoptotic and autophagic cell death. Cell Death Dis 5:e1147. doi:10.1038/cddis.2014.123
Deng H, Mi MT (2016) Resveratrol attenuates Aβ25-35 caused neurotoxicity by inducing autophagy through the TyrRS-PARP1-SIRT1 signaling pathway. Neurochem Res 41:2367–2379. doi:10.1007/s11064-016-1950-9
Venigalla M, Sonego S, Gyengesi E, Sharman MJ, Münch G (2016) Novel promising therapeutics against chronic neuroinflammation and neurodegeneration in Alzheimer's disease. Neurochem Int 95:63–74. doi:10.1016/j.neuint.2015.10.011
Zeng W, Zhang W, Lu F, Gao L, Gao G (2016) Resveratrol attenuates MPP+-induced mitochondrial dysfunction and cell apoptosis via AKT/GSK-3β pathway in SN4741 cells. Neurosci Lett. doi:10.1016/j.neulet.2016.11.054
Sharma M, Gupta YK (2002) Chronic treatment with trans-resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life Sci 71:2489–2498
Schmatz R, Mazzanti CM, Spanevello R, Stefanello N, Gutierres J, Corrêa M, da Rosa MM, Rubin MA et al (2009) Resveratrol prevents memory deficits and the increase in acetylcholinesterase activity in streptozotocin-induced diabetic rats. Eur J Pharmacol 610:42–48. doi:10.1016/j.ejphar.2009.03.032
Bagatini PB, Xavier LL, Bertoldi K, Moysés F, Lovatel G, Neves LT, Barbosa S, Saur L et al (2017) An evaluation of aversive memory and hippocampal oxidative status in streptozotocin-induced diabetic rats treated with resveratrol. Neurosci Lett 636:184–189. doi:10.1016/j.neulet.2016.10.059
Gocmez SS, Gacar N, Utkan T, Gacar G, Scarpace PJ, Tumer N (2016) Protective effects of resveratrol on aging-induced cognitive impairment in rats. Neurobiol Learn Mem 131:131–136. doi:10.1016/j.nlm.2016.03.022
Wang R, Zhang Y, Li J, Zhang C (2016) Resveratrol ameliorates spatial learning memory impairment induced by Aβ1-42 in rats. Neuroscience. doi:10.1016/j.neuroscience.2016.08.051
Hurley LL, Tizabi Y (2013) Neuroinflammation, neurodegeneration, and depression. Neurotox Res 23:131–144. doi:10.1007/s12640-012-9348-1
Wang X, Xie Y, Zhang T, Bo S, Bai X, Liu H, Li T, Liu S et al (2016) Resveratrol reverses chronic restraint stress-induced depression-like behaviour: involvement of BDNF level, ERK phosphorylation and expression of Bcl-2 and Bax in rats. Brain Res Bull 125:134–143. doi:10.1016/j.brainresbull.2016.06.014
Ge JF, Xu YY, Qin G, Cheng JQ, Chen FH (2016) Resveratrol ameliorates the anxiety- and depression-like behavior of subclinical hypothyroidism rat: possible involvement of the HPT axis, HPA axis, and Wnt/β-catenin pathway. Front Endocrinol (Lausanne) 7:44. doi:10.3389/fendo.2016.00044
Liu S, Li T, Liu H, Wang X, Bo S, Xie Y, Bai X, Wu L et al (2016) Resveratrol exerts antidepressant properties in the chronic unpredictable mild stress model through the regulation of oxidative stress and mTOR pathway in the rat hippocampus and prefrontal cortex. Behav Brain Res 302:191–199. doi:10.1016/j.bbr.2016.01.037
Tizabi Y (2016) Duality of antidepressants and neuroprotectants. Neurotox Res 30:1–13. doi:10.1007/s12640-015-9577-1
Wang Q, Xu J, Rottinghaus GE, Simonyi A, Lubahn D, Sun GY, Sun AY (2002) Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res 958:439–447
Dinuzzo M, Mangia S, Maraviglia B, Giove F (2012) The role of astrocytic glycogen in supporting the energetics of neuronal activity. Neurochem Res 37:2432–2438. doi:10.1007/s11064-012-0802-5
Falkowska A, Gutowska I, Goschorska M, Nowacki P, Chlubek D, Baranowska-Bosiacka I (2015) Energy metabolism of the brain, including the cooperation between astrocytes and neurons, especially in the context of glycogen metabolism. Int J Mol Sci 16:25959–25981. doi:10.3390/ijms161125939
de Oliveira MR (2016) The dietary components carnosic acid and carnosol as neuroprotective agents: a mechanistic view. Mol Neurobiol 53:6155–6168
Valero T (2014) Mitochondrial biogenesis: pharmacological approaches. Curr Pharm Des 20:5507–5509
Gibellini L, Bianchini E, De Biasi S, Nasi M, Cossarizza A, Pinti M (2015) Natural compounds modulating mitochondrial functions. Evid Based Complement Alternat Med 2015:527209. doi:10.1155/2015/527209
Tellone E, Galtieri A, Russo A, Giardina B, Ficarra S (2015) Resveratrol: a focus on several neurodegenerative diseases. Oxidative Med Cell Longev 2015:392169. doi:10.1155/2015/392169
Papa S, Martino PL, Capitanio G, Gaballo A, De Rasmo D, Signorile A, Petruzzella V (2012) The oxidative phosphorylation system in mammalian mitochondria. Adv Exp Med Biol 942:3–37. doi:10.1007/978-94-007-2869-1_1
Chaban Y, Boekema EJ, Dudkina NV (2014) Structures of mitochondrial oxidative phosphorylation supercomplexes and mechanisms for their stabilisation. Biochim Biophys Acta 1837:418–426. doi:10.1016/j.bbabio.2013.10.004
Russell AP, Foletta VC, Snow RJ, Wadley GD (2014) Skeletal muscle mitochondria: a major player in exercise, health and disease. Biochim Biophys Acta 1840:1276–1284. doi:10.1016/j.bbagen.2013.11.016
Zhou L, Chen P, Peng Y, Ouyang R (2016) Role of oxidative stress in the neurocognitive dysfunction of obstructive sleep apnea syndrome. Oxidative Med Cell Longev 2016:9626831
Islam MT (2017) Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol Res 39:73–82
Brown GC, Bal-Price A (2003) Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Mol Neurobiol 27:325–355
Naoi M, Maruyama W, Shamoto-Nagai M, Yi H, Akao Y, Tanaka M (2005) Oxidative stress in mitochondria: decision to survival and death of neurons in neurodegenerative disorders. Mol Neurobiol 31:81–93
Kasote DM, Hegde MV, Katyare SS (2013) Mitochondrial dysfunction in psychiatric and neurological diseases: cause(s), consequence(s), and implications of antioxidant therapy. Biofactors 39:392–406
Cadonic C, Sabbir MG, Albensi BC (2016) Mechanisms of mitochondrial dysfunction in Alzheimer's disease. Mol Neurobiol 53:6078–6090. doi:10.1007/s12035-015-9515-5
Hu H, Tan CC, Tan L, Yu JT (2016) A mitocentric view of Alzheimer's disease. Mol Neurobiol. doi:10.1007/s12035-016-0117-7
Balog J, Mehta SL, Vemuganti R (2016) Mitochondrial fission and fusion in secondary brain damage after CNS insults. J Cereb Blood Flow Metab 36:2022–2033
Bergman O, Ben-Shachar D (2016) Mitochondrial oxidative phosphorylation system (OXPHOS) deficits in schizophrenia: possible interactions with cellular processes. Can J Psychiatr 61:457–469. doi:10.1177/0706743716648290
Zulian A, Schiavone M, Giorgio V, Bernardi P (2016) Forty years later: mitochondria as therapeutic targets in muscle diseases. Pharmacol Res 113(Pt A):563–573. doi:10.1016/j.phrs.2016.09.043
Luo C, Ikegaya Y, Koyama R (2016) Microglia and neurogenesis in the epileptic dentate gyrus. Neurogenesis (Austin) 3:e1235525
Gonçalves JT, Schafer ST, Gage FH (2016) Adult neurogenesis in the hippocampus: from stem cells to behavior. Cell 167:897–914. doi:10.1016/j.cell.2016.10.021
Raefsky SM, Mattson MP (2016) Adaptive responses of neuronal mitochondria to bioenergetic challenges: roles in neuroplasticity and disease resistance. Free Radic Biol Med 102:203–216. doi:10.1016/j.freeradbiomed.2016.11.045
Mattson MP (2007) Mitochondrial regulation of neuronal plasticity. Neurochem Res 32:707–715
Atamna H, Mackey J, Dhahbi JM (2012) Mitochondrial pharmacology: electron transport chain bypass as strategies to treat mitochondrial dysfunction. Biofactors 38:158–166
Gruber J, Fong S, Chen CB, Yoong S, Pastorin G, Schaffer S, Cheah I, Halliwell B (2013) Mitochondria-targeted antioxidants and metabolic modulators as pharmacological interventions to slow ageing. Biotechnol Adv 31:563–592. doi:10.1016/j.biotechadv.2012.09.005
de Oliveira MR, Ferreira GC, Schuck PF, Dal Bosco SM (2015) Role for the PI3K/Akt/Nrf2 signaling pathway in the protective effects of carnosic acid against methylglyoxal-induced neurotoxicity in SH-SY5Y neuroblastoma cells. Chem Biol Interact 242:396–406. doi:10.1016/j.cbi.2015.11.003
de Oliveira MR, Nabavi SF, Habtemariam S, Erdogan Orhan I, Daglia M, Nabavi SM (2015) The effects of baicalein and baicalin on mitochondrial function and dynamics: a review. Pharmacol Res 100:296–308. doi:10.1016/j.phrs.2015.08.021
de Oliveira MR, Nabavi SM, Braidy N, Setzer WN, Ahmed T, Nabavi SF (2016) Quercetin and the mitochondria: a mechanistic view. Biotechnol Adv 34:532–549. doi:10.1016/j.biotechadv.2015.12.014
de Oliveira MR, Jardim FR, Setzer WN, Nabavi SM, Nabavi SF (2016) Curcumin, mitochondrial biogenesis, and mitophagy: exploring recent data and indicating future needs. Biotechnol Adv 34:813–826. doi:10.1016/j.biotechadv.2016.04.004
Oliveira MR, Nabavi SF, Daglia M, Rastrelli L, Nabavi SM (2016) Epigallocatechin gallate and mitochondria—a story of life and death. Pharmacol Res 104:70–85. doi:10.1016/j.phrs.2015.12.027
de Oliveira MR, Peres A, Ferreira GC, Schuck PF, Gama CS, Bosco SM (2016) Carnosic acid protects mitochondria of human neuroblastoma SH-SY5Y cells exposed to paraquat through activation of the Nrf2/HO-1Axis. Mol Neurobiol. doi:10.1007/s12035-016-0100-3
de Oliveira MR, Peres A, Ferreira GC, Schuck PF, Bosco SM (2016) Carnosic acid affords mitochondrial protection in chlorpyrifos-treated Sh-Sy5y cells. Neurotox Res 30:367–379. doi:10.1007/s12640-016-9620-x
de Oliveira MR, Ferreira GC, Schuck PF (2016) Protective effect of carnosic acid against paraquat-induced redox impairment and mitochondrial dysfunction in SH-SY5Y cells: role for PI3K/Akt/Nrf2 pathway. Toxicol in Vitro 32:41–54. doi:10.1016/j.tiv.2015.12.005
Ueta CB, Gomes KS, Ribeiro MA, Mochly-Rosen D, Ferreira JC (2016) Disruption of mitochondrial quality control in peripheral artery disease: new therapeutic opportunities. Pharmacol Res 115:96–106. doi:10.1016/j.phrs.2016.11.016
Sandoval-Acuña C, Ferreira J, Speisky H (2014) Polyphenols and mitochondria: an update on their increasingly emerging ROS-scavenging independent actions. Arch Biochem Biophys 559:75–90. doi:10.1016/j.abb.2014.05.017
Jiang T, Sun Q, Chen S (2016) Oxidative stress: a major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson's disease and Alzheimer's disease. Prog Neurobiol 147:1–19. doi:10.1016/j.pneurobio.2016.07.005
Zhang Y, Xu H (2016) Translational regulation of mitochondrial biogenesis. Biochem Soc Trans 44:1717–1724. doi:10.1042/BST20160071C
Lee H, Yoon Y (2016) Mitochondrial fission and fusion. Biochem Soc Trans 44:1725–1735. doi:10.1042/BST20160129
Green DR, Galluzzi L, Kroemer G (2014) Metabolic control of cell death. Science 345:1250256. doi:10.1126/science.1250256
Picard M, Wallace DC, Burelle Y (2016) The rise of mitochondria in medicine. Mitochondrion 30:105–116. doi:10.1016/j.mito.2016.07.003
Zorov DB, Juhaszova M, Sollott SJ (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94:909–950. doi:10.1152/physrev.00026.2013
Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, Uchida K, Arimura K et al (1999) Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res 85:357–363
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552:335–344
de Oliveira MR, Jardim FR (2016) Cocaine and mitochondria-related signaling in the brain: a mechanistic view and future directions. Neurochem Int 92:58–66. doi:10.1016/j.neuint.2015.12.006
de Oliveira MR (2015) Vitamin A and retinoids as mitochondrial toxicants. Oxidative Med Cell Longev 2015:140267. doi:10.1155/2015/140267
Lu SC (2013) Glutathione synthesis. Biochim Biophys Acta 1830:3143–3153. doi:10.1016/j.bbagen.2012.09.008
Flynn JM, Melov S (2013) SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radic Biol Med 62:4–12. doi:10.1016/j.freeradbiomed.2013.05.027
Morris G, Anderson G, Dean O, Berk M, Galecki P, Martin-Subero M, Maes M (2014) The glutathione system: a new drug target in neuroimmune disorders. Mol Neurobiol 50:1059–1084. doi:10.1007/s12035-014-8705-x
Barrera G, Gentile F, Pizzimenti S, Canuto RA, Daga M, Arcaro A, Cetrangolo GP, Lepore A et al (2016) Mitochondrial dysfunction in cancer and neurodegenerative diseases: spotlight on fatty acid oxidation and lipoperoxidation products. Antioxidants (Basel) 5:7. doi:10.3390/antiox5010007
Edmondson DE (2014) Hydrogen peroxide produced by mitochondrial monoamine oxidase catalysis: biological implications. Curr Pharm Des 20:155–160
Veal EA, Day AM, Morgan BA (2007) Hydrogen peroxide sensing and signaling. Mol Cell 26:1–14
Pinho RA, Andrades ME, Oliveira MR, Pirola AC, Zago MS, Silveira PC, Dal-Pizzol F, Moreira JC (2006) Imbalance in SOD/CAT activities in rat skeletal muscles submitted to treadmill training exercise. Cell Biol Int 30:848–853
Andrades M, Ritter C, de Oliveira MR, Streck EL, Fonseca Moreira JC, Dal-Pizzol F (2011) Antioxidant treatment reverses organ failure in rat model of sepsis: role of antioxidant enzymes imbalance, neutrophil infiltration, and oxidative stress. J Surg Res 167:e307–e313. doi:10.1016/j.jss.2009.08.005
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658
Gutteridge JM (1994) Hydroxyl radicals, iron, oxidative stress, and neurodegeneration. Ann N Y Acad Sci 738:201–213
Ghafourifar P, Cadenas E (2005) Mitochondrial nitric oxide synthase. Trends Pharmacol Sci 26:190–195
Finocchietto PV, Franco MC, Holod S, Gonzalez AS, Converso DP, Antico Arciuch VG, Serra MP, Poderoso JJ et al (2009) Mitochondrial nitric oxide synthase: a masterpiece of metabolic adaptation, cell growth, transformation, and death. Exp Biol Med (Maywood) 234:1020–1028. doi:10.3181/0902-MR-81
Valez V, Cassina A, Batinic-Haberle I, Kalyanaraman B, Ferrer-Sueta G, Radi R (2013) Peroxynitrite formation in nitric oxide-exposed submitochondrial particles: detection, oxidative damage and catalytic removal by Mn-porphyrins. Arch Biochem Biophys 529:45–54. doi:10.1016/j.abb.2012.10.012
Campolo N, Bartesaghi S, Radi R (2014) Metal-catalyzed protein tyrosine nitration in biological systems. Redox Rep 19:221–231. doi:10.1179/1351000214Y.0000000099
Alvarez B, Radi R (2003) Peroxynitrite reactivity with amino acids and proteins. Amino Acids 25:295–311
Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM (2007) Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci 8:766–775
De Oliveira MR, Oliveira MW, Da Rocha RF, Moreira JC (2009) Vitamin A supplementation at pharmacological doses induces nitrosative stress on the hypothalamus of adult Wistar rats. Chem Biol Interact 180:407–413. doi:10.1016/j.cbi.2009.02.006
de Oliveira MR, da Rocha RF, Stertz L, Fries GR, de Oliveira DL, Kapczinski F, Moreira JC (2011) Total and mitochondrial nitrosative stress, decreased brain-derived neurotrophic factor (BDNF) levels and glutamate uptake, and evidence of endoplasmic reticulum stress in the hippocampus of vitamin A-treated rats. Neurochem Res 36:506–517. doi:10.1007/s11064-010-0372-3
Swomley AM, Butterfield DA (2015) Oxidative stress in Alzheimer disease and mild cognitive impairment: evidence from human data provided by redox proteomics. Arch Toxicol 89:1669–1680. doi:10.1007/s00204-015-1556-z
Yao D, Gu Z, Nakamura T, Shi ZQ, Ma Y, Gaston B, Palmer LA, Rockenstein EM et al (2004) Nitrosative stress linked to sporadic Parkinson's disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci U S A 101:10810–10814
Gu Z, Nakamura T, Yao D, Shi ZQ, Lipton SA (2005) Nitrosative and oxidative stress links dysfunctional ubiquitination to Parkinson's disease. Cell Death Differ 12:1202–1204
Pal R, Miranda M, Narayan M (2011) Nitrosative stress-induced Parkinsonian Lewy-like aggregates prevented through polyphenolic phytochemical analog intervention. Biochem Biophys Res Commun 404:324–329. doi:10.1016/j.bbrc.2010.11.117
Sorolla MA, Rodríguez-Colman MJ, Vall-llaura N, Tamarit J, Ros J, Cabiscol E (2012) Protein oxidation in Huntington disease. Biofactors 38:173–185
Tasset I, Sánchez-López F, Agüera E, Fernández-Bolaños R, Sánchez FM, Cruz-Guerrero A, Gascón-Luna F, Túnez I (2012) NGF and nitrosative stress in patients with Huntington's disease. J Neurol Sci 315:133–136. doi:10.1016/j.jns.2011.12.014
Bossy-Wetzel E, Schwarzenbacher R, Lipton SA (2004) Molecular pathways to neurodegeneration. Nat Med 10(Suppl):S2–S9. doi:10.1038/nm1067
Anderson G, Maes M, Berk M (2013) Schizophrenia is primed for an increased expression of depression through activation of immuno-inflammatory, oxidative and nitrosative stress, and tryptophan catabolite pathways. Prog Neuro-Psychopharmacol Biol Psychiatry 42:101–114. doi:10.1016/j.pnpbp.2012.07.016
Moylan S, Berk M, Dean OM, Samuni Y, Williams LJ, O'Neil A, Hayley AC, Pasco JA et al (2014) Oxidative & nitrosative stress in depression: why so much stress? Neurosci Biobehav Rev 45:46–62. doi:10.1016/j.neubiorev.2014.05.007
Morris G, Walder K, Puri BK, Berk M, Maes M (2016) The deleterious effects of oxidative and nitrosative stress on palmitoylation, membrane lipid rafts and lipid-based cellular signalling: new drug targets in neuroimmune disorders. Mol Neurobiol 53:4638–4658. doi:10.1007/s12035-015-9392-y
Delhalle S, Deregowski V, Benoit V, Merville MP, Bours V (2002) NF-kappaB-dependent MnSOD expression protects adenocarcinoma cells from TNF-alpha-induced apoptosis. Oncogene 21:3917–3924
Esteras N, Dinkova-Kostova AT, Abramov AY (2016) Nrf2 activation in the treatment of neurodegenerative diseases: a focus on its role in mitochondrial bioenergetics and function. Biol Chem 397:383–400. doi:10.1515/hsz-2015-0295
Hayes JD, Dinkova-Kostova AT (2014) The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci 39:199–218. doi:10.1016/j.tibs.2014.02.002
Piantadosi CA, Suliman HB (2012) Transcriptional control of mitochondrial biogenesis and its interface with inflammatory processes. Biochim Biophys Acta 1820:532–541. doi:10.1016/j.bbagen.2012.01.003
Denzer I, Münch G, Friedland K (2016) Modulation of mitochondrial dysfunction in neurodegenerative diseases via activation of nuclear factor erythroid-2-related factor 2 by food-derived compounds. Pharmacol Res 103:80–94. doi:10.1016/j.phrs.2015.11.019
Kansanen E, Kuosmanen SM, Leinonen H, Levonen AL (2013) The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol 1:45–49. doi:10.1016/j.redox.2012.10.001
Zhang M, An C, Gao Y, Leak RK, Chen J, Zhang F (2013) Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog Neurobiol 100:30–47. doi:10.1016/j.pneurobio.2012.09.003
Gilmore TD (2006) Introduction to NF-kappaB: players, pathways, perspectives. Oncogene 25:6680–6684
Lin CY, Chen JH, Fu RH, Tsai CW (2014) Induction of Pi form of glutathione S-transferase by carnosic acid is mediated through PI3K/Akt/NF-κB pathway and protects against neurotoxicity. Chem Res Toxicol 27:1958–1966. doi:10.1021/tx5003063
Sheehan D, Meade G, Foley VM, Dowd CA (2001) Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J 360:1–16
Nguyen T, Nioi P, Pickett CB (2009) The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 284:13291–13295. doi:10.1074/jbc.R900010200
Ghosh S, Dass JF (2016) Study of pathway cross-talk interactions with NF-κB leading to its activation via ubiquitination or phosphorylation: a brief review. Gene 584:97–109. doi:10.1016/j.gene.2016.03.008
Hock MB, Kralli A (2009) Transcriptional control of mitochondrial biogenesis and function. Annu Rev Physiol 71:177–203. doi:10.1146/annurev.physiol.010908.163119
Jornayvaz FR, Shulman GI (2010) Regulation of mitochondrial biogenesis. Essays Biochem 47:69–84. doi:10.1042/bse0470069
Ungvari Z, Sonntag WE, de Cabo R, Baur JA, Csiszar A (2011) Mitochondrial protection by resveratrol. Exerc Sport Sci Rev 39:128–132. doi:10.1097/JES.0b013e3182141f80
Scarpulla RC (2011) Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta 1813:1269–1278. doi:10.1016/j.bbamcr.2010.09.019
Schreiber SN, Emter R, Hock MB, Knutti D, Cardenas J, Podvinec M, Oakeley EJ, Kralli A (2004) The estrogen-related receptor alpha (ERRalpha) functions in PPARgamma coactivator 1alpha (PGC-1alpha)-induced mitochondrial biogenesis. Proc Natl Acad Sci U S A 101:6472–6477
Virbasius CA, Virbasius JV, Scarpulla RC (1993) NRF-1, an activator involved in nuclear-mitochondrial interactions, utilizes a new DNA-binding domain conserved in a family of developmental regulators. Genes Dev 7:2431–2445
Schreiber SN, Knutti D, Brogli K, Uhlmann T, Kralli A (2003) The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor alpha (ERRalpha). J Biol Chem 278:9013–9018
Anderson R, Prolla T (2009) PGC-1alpha in aging and anti-aging interventions. Biochim Biophys Acta 1790:1059–1066. doi:10.1016/j.bbagen.2009.04.005
Gleyzer N, Vercauteren K, Scarpulla RC (2005) Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol 25:1354–1366
Fisher RP, Clayton DA (1988) Purification and characterization of human mitochondrial transcription factor 1. Mol Cell Biol 8:3496–3509
Canugovi C, Maynard S, Bayne AC, Sykora P, Tian J, de Souza-Pinto NC, Croteau DL, Bohr VA (2010) The mitochondrial transcription factor A functions in mitochondrial base excision repair. DNA Repair (Amst) 9:1080–1089. doi:10.1016/j.dnarep.2010.07.009
Ekstrand MI, Falkenberg M, Rantanen A, Park CB, Gaspari M, Hultenby K, Rustin P, Gustafsson CM et al (2004) Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum Mol Genet 13:935–944
Picca A, Lezza AM (2015) Regulation of mitochondrial biogenesis through TFAM-mitochondrial DNA interactions: useful insights from aging and calorie restriction studies. Mitochondrion 25:67–75. doi:10.1016/j.mito.2015.10.001
Nemoto S, Fergusson MM, Finkel T (2005) SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J Biol Chem 280:16456–16460
Sugden MC, Caton PW, Holness MJ (2010) PPAR control: it's SIRTainly as easy as PGC. J Endocrinol 204:93–104. doi:10.1677/JOE-09-0359
Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P et al (2009) AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458:1056–1060. doi:10.1038/nature07813
Paoli A, Bianco A, Damiani E, Bosco G (2014) Ketogenic diet in neuromuscular and neurodegenerative diseases. Biomed Res Int 2014:474296. doi:10.1155/2014/474296
Wang KZ, Zhu J, Dagda RK, Uechi G, Cherra SJ 3rd, Gusdon AM, Balasubramani M, Chu CT (2014) ERK-mediated phosphorylation of TFAM downregulates mitochondrial transcription: implications for Parkinson's disease. Mitochondrion 17:132–140. doi:10.1016/j.mito.2014.04.008
Banerjee K, Munshi S, Frank DE, Gibson GE (2015) Abnormal glucose metabolism in Alzheimer's disease: relation to autophagy/mitophagy and therapeutic approaches. Neurochem Res 40:2557–2569. doi:10.1007/s11064-015-1631-0
de Olivera MR (2016) Evidence for genistein as a mitochondriotropic molecule. Mitochondrion 29:35–44. doi:10.1016/j.mito.2016.05.005
Schmitt CA, Heiss EH, Dirsch VM (2010) Effect of resveratrol on endothelial cell function: molecular mechanisms. Biofactors 36:342–349
Dragone T, Cianciulli A, Calvello R, Porro C, Trotta T, Panaro MA (2014) Resveratrol counteracts lipopolysaccharide-mediated microglial inflammation by modulating a SOCS-1 dependent signaling pathway. Toxicol in Vitro 28:1126–1135. doi:10.1016/j.tiv.2014.05.005
Sun D, Yue Q, Guo W, Li T, Zhang J, Li G, Liu Z, Sun J (2015) Neuroprotection of resveratrol against neurotoxicity induced by methamphetamine in mouse mesencephalic dopaminergic neurons. Biofactors 41:252–260
Lopez MS, Dempsey RJ, Vemuganti R (2015) Resveratrol neuroprotection in stroke and traumatic CNS injury. Neurochem Int 89:75–82. doi:10.1016/j.neuint.2015.08.009
Wang Y, Jiang Y, Fan X, Tan H, Zeng H, Wang Y, Chen P, Huang M et al (2015) Hepato-protective effect of resveratrol against acetaminophen-induced liver injury is associated with inhibition of CYP-mediated bioactivation and regulation of SIRT1-p53 signaling pathways. Toxicol Lett 236:82–89. doi:10.1016/j.toxlet.2015.05.001
Steiner N, Balez R, Karunaweera N, Lind JM, Münch G, Ooi L (2016) Neuroprotection of Neuro2a cells and the cytokine suppressive and anti-inflammatory mode of action of resveratrol in activated RAW264.7 macrophages and C8-B4 microglia. Neurochem Int 5:46–54. doi:10.1016/j.neuint.2015.10.013
Arun S, Liu L, Donmez G (2016) Mitochondrial biology and neurological diseases. Curr Neuropharmacol 14:143–154
Erdogan CS, Vang O (2016) Challenges in analyzing the biological effects of resveratrol. Nutrients 8:353. doi:10.3390/nu8060353
Morbidelli L (2016) Polyphenol-based nutraceuticals for the control of angiogenesis: analysis of the critical issues for human use. Pharmacol Res 111:384–393. doi:10.1016/j.phrs.2016.07.011
Weiskirchen S, Weiskirchen R (2016) Resveratrol: how much wine do you have to drink to stay healthy? Adv Nutr 7:706–718. doi:10.3945/an.115.011627
Rowlands BD, Lau CL, Ryall JG, Thomas DS, Klugmann M, Beart PM, Rae CD (2015) Silent information regulator 1 modulator resveratrol increases brain lactate production and inhibits mitochondrial metabolism, whereas SRT1720 increases oxidative metabolism. J Neurosci Res 93:1147–1156. doi:10.1002/jnr.23570
Varoni EM, Lo Faro AF, Sharifi-Rad J, Iriti M (2016) Anticancer molecular mechanisms of resveratrol. Front Nutr 3:8. doi:10.3389/fnut.2016.00008
Kairisalo M, Bonomo A, Hyrskyluoto A, Mudò G, Belluardo N, Korhonen L, Lindholm D (2011) Resveratrol reduces oxidative stress and cell death and increases mitochondrial antioxidants and XIAP in PC6.3-cells. Neurosci Lett 488:263–266. doi:10.1016/j.neulet.2010.11.042
Sheu SJ, Liu NC, Ou CC, Bee YS, Chen SC, Lin HC, Chan JY (2013) Resveratrol stimulates mitochondrial bioenergetics to protect retinal pigment epithelial cells from oxidative damage. Invest Ophthalmol Vis Sci 54:6426–6438. doi:10.1167/iovs.13-12024
Mudò G, Mäkelä J, Di Liberto V, Tselykh TV, Olivieri M, Piepponen P, Eriksson O, Mälkiä A et al (2012) Transgenic expression and activation of PGC-1α protect dopaminergic neurons in the MPTP mouse model of Parkinson's disease. Cell Mol Life Sci 69:1153–1165. doi:10.1007/s00018-011-0850-z
Narayanan SV, Dave KR, Saul I, Perez-Pinzon MA (2015) Resveratrol preconditioning protects against cerebral ischemic injury via nuclear erythroid 2-related factor 2. Stroke 46:1626–1632. doi:10.1161/STROKEAHA.115.008921
Bellaver B, Bobermin LD, Souza DG, Rodrigues MD, de Assis AM, Wajner M, Gonçalves CA, Souza DO et al (2016) Signaling mechanisms underlying the glioprotective effects of resveratrol against mitochondrial dysfunction. Biochim Biophys Acta 1862:1827–1838. doi:10.1016/j.bbadis.2016.06.018
Robb EL, Winkelmolen L, Visanji N, Brotchie J, Stuart JA (2008) Dietary resveratrol administration increases MnSOD expression and activity in mouse brain. Biochem Biophys Res Commun 372:254–259. doi:10.1016/j.bbrc.2008.05.028
Zhao H, Niu Q, Li X, Liu T, Xu Y, Han H, Wang W, Fan N et al (2012) Long-term resveratrol consumption protects ovariectomized rats chronically treated with D-galactose from developing memory decline without effects on the uterus. Brain Res 1467:67–80. doi:10.1016/j.brainres.2012.05.040
Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B et al (2010) Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer's disease neurons. J Alzheimers Dis 20:S609–S631. doi:10.3233/JAD-2010-100564
Yu L, Yang SJ (2010) AMP-activated protein kinase mediates activity-dependent regulation of peroxisome proliferator-activated receptor gamma coactivator-1alpha and nuclear respiratory factor 1 expression in rat visual cortical neurons. Neuroscience 169:23–38. doi:10.1016/j.neuroscience.2010.04.063
Chen S, Fan Q, Li A, Liao D, Ge J, Laties AM, Zhang X (2013) Dynamic mobilization of PGC-1α mediates mitochondrial biogenesis for the protection of RGC-5 cells by resveratrol during serum deprivation. Apoptosis 18:786–799. doi:10.1007/s10495-013-0837-3
Cao K, Zheng A, Xu J, Li H, Liu J, Peng Y, Long J, Zou X et al (2014) AMPK activation prevents prenatal stress-induced cognitive impairment: modulation of mitochondrial content and oxidative stress. Free Radic Biol Med 75:156–166. doi:10.1016/j.freeradbiomed.2014.07.029
Peng K, Tao Y, Zhang J, Wang J, Ye F, Dan G, Zhao Y, Cai Y et al (2016) Resveratrol regulates mitochondrial biogenesis and fission/fusion to attenuate rotenone-induced neurotoxicity. Oxidative Med Cell Longev 2016:6705621. doi:10.1155/2016/6705621
Valenti D, de Bari L, de Rasmo D, Signorile A, Henrion-Caude A, Contestabile A, Vacca RA (2016) The polyphenols resveratrol and epigallocatechin-3-gallate restore the severe impairment of mitochondria in hippocampal progenitor cells from a Down syndrome mouse model. Biochim Biophys Acta 1862:1093–1104. doi:10.1016/j.bbadis.2016.03.003
Naia L, Rosenstock TR, Oliveira AM, Oliveira-Sousa SI, Caldeira GL, Carmo C, Laço MN, Hayden MR et al (2016) Comparative mitochondrial-based protective effects of resveratrol and nicotinamide in Huntington's disease models. Mol Neurobiol. doi:10.1007/s12035-016-0048-3
Mancuso R, del Valle J, Modol L, Martinez A, Granado-Serrano AB, Ramirez-Núñez O, Pallás M, Portero-Otin M et al (2014) Resveratrol improves motoneuron function and extends survival in SOD1(G93A) ALS mice. Neurotherapeutics 11:419–432. doi:10.1007/s13311-013-0253-y
Palomera-Avalos V, Griñán-Ferré C, Puigoriol-Ilamola D, Camins A, Sanfeliu C, Canudas AM, Pallàs M (2016) Resveratrol protects SAMP8 brain under metabolic stress: focus on mitochondrial function and Wnt pathway. Mol Neurobiol. doi:10.1007/s12035-016-9770-0
Kairisalo M, Korhonen L, Blomgren K, Lindholm D (2007) X-linked inhibitor of apoptosis protein increases mitochondrial antioxidants through NF-kappaB activation. Biochem Biophys Res Commun 364:138–144
Jang JH, Surh YJ (2003) Protective effect of resveratrol on beta-amyloid-induced oxidative PC12 cell death. Free Radic Biol Med 34:1100–1110
Miao L, St Clair DK (2009) Regulation of superoxide dismutase genes: implications in disease. Free Radic Biol Med 47:344–356. doi:10.1016/j.freeradbiomed.2009.05.018
Lu Z, Xu X, Hu X, Fassett J, Zhu G, Tao Y, Li J, Huang Y et al (2010) PGC-1 alpha regulates expression of myocardial mitochondrial antioxidants and myocardial oxidative stress after chronic systolic overload. Antioxid Redox Signal 13:1011–1022. doi:10.1089/ars.2009.2940
Nijland PG, Witte ME, van het Hof B, van der Pol S, Bauer J, Lassmann H, van der Valk P, de Vries HE et al (2014) Astroglial PGC-1alpha increases mitochondrial antioxidant capacity and suppresses inflammation: implications for multiple sclerosis. Acta Neuropathol Commun 2:170. doi:10.1186/s40478-014-0170-2
Quincozes-Santos A, Bobermin LD, Tramontina AC, Wartchow KM, Tagliari B, Souza DO, Wyse AT, Gonçalves CA (2014) Oxidative stress mediated by NMDA, AMPA/KA channels in acute hippocampal slices: neuroprotective effect of resveratrol. Toxicol in Vitro 28:544–551. doi:10.1016/j.tiv.2013.12.021
Wang R, Liu YY, Liu XY, Jia SW, Zhao J, Cui D, Wang L (2014) Resveratrol protects neurons and the myocardium by reducing oxidative stress and ameliorating mitochondria damage in a cerebral ischemia rat model. Cell Physiol Biochem 34:854–864. doi:10.1159/000366304
Henry-Mowatt J, Dive C, Martinou JC, James D (2004) Role of mitochondrial membrane permeabilization in apoptosis and cancer. Oncogene 23:2850–2860
Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, Perez-Pinzon MA (2009) Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience 159:993–1002. doi:10.1016/j.neuroscience.2009.01.017
Guo C, Yang L, Wan CX, Xia YZ, Zhang C, Chen MH, Wang ZD, Li ZR et al (2016) Anti-neuroinflammatory effect of Sophoraflavanone G from Sophora alopecuroides in LPS-activated BV2 microglia by MAPK, JAK/STAT and Nrf2/HO-1 signaling pathways. Phytomedicine 23:1629–1637. doi:10.1016/j.phymed.2016.10.007
Kim CS, Choi HS, Joe Y, Chung HT, Yu R (2016) Induction of heme oxygenase-1 with dietary quercetin reduces obesity-induced hepatic inflammation through macrophage phenotype switching. Nutr Res Pract 10:623–628
Choi YK, Park JH, Baek YY, Won MH, Jeoung D, Lee H, Ha KS, Kwon YG et al (2016) Carbon monoxide stimulates astrocytic mitochondrial biogenesis via L-type Ca2+ channel-mediated PGC-1α/ERRα activation. Biochem Biophys Res Commun 479:297–304. doi:10.1016/j.bbrc.2016.09.063
Yu J, Shi J, Wang D, Dong S, Zhang Y, Wang M, Gong L, Fu Q et al (2016) Heme oxygenase-1/carbon monoxide-regulated mitochondrial dynamic equilibrium contributes to the attenuation of endotoxin-induced acute lung injury in rats and in lipopolysaccharide-activated macrophages. Anesthesiology 125:1190–1201
Holmström KM, Baird L, Zhang Y, Hargreaves I, Chalasani A, Land JM, Stanyer L, Yamamoto M et al (2013) Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol Open 2:761–770. doi:10.1242/bio.20134853
de Oliveira MR, Fürstenau CR, de Souza IC, da Costa Ferreira G (2016) Tanshinone I attenuates the effects of a challenge with H2O2 on the functions of tricarboxylic acid cycle and respiratory chain in SH-SY5Y cells. Mol Neurobiol doi. doi:10.1007/s12035-016-0267-7
de Oliveira MR, Schuck PF, Bosco SM (2016) Tanshinone I induces mitochondrial protection through an Nrf2-dependent mechanism in paraquat-treated human neuroblastoma SH-SY5Y cells. Mol Neurobiol. doi:10.1007/s12035-016-0009-x
de Oliveira MR, Peres A, Gama CS, Bosco SM (2016) Pinocembrin provides mitochondrial protection by the activation of the Erk1/2-Nrf2 signaling pathway in SH-SY5Y neuroblastoma cells exposed to paraquat. Mol Neurobiol. doi:10.1007/s12035-016-0135-5
Cerveny KL, Tamura Y, Zhang Z, Jensen RE, Sesaki H (2007) Regulation of mitochondrial fusion and division. Trends Cell Biol 17:563–569. doi:10.1016/j.tcb.2007.08.006
Westermann B (2010) Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 11:872–884. doi:10.1038/nrm3013
Morris-Blanco KC, Cohan CH, Neumann JT, Sick TJ, Perez-Pinzon MA (2014) Protein kinase C epsilon regulates mitochondrial pools of Nampt and NAD following resveratrol and ischemic preconditioning in the rat cortex. J Cereb Blood Flow Metab 34:1024–1032. doi:10.1038/jcbfm.2014.51
Peng Y, Liu J, Shi L, Tang Y, Gao D, Long J, Liu J (2016) Mitochondrial dysfunction precedes depression of AMPK/AKT signaling in insulin resistance induced by high glucose in primary cortical neurons. J Neurochem 137:701–713. doi:10.1111/jnc.13563
Valenti D, Tullo A, Caratozzolo MF, Merafina RS, Scartezzini P, Marra E, Vacca RA (2010) Impairment of F1F0-ATPase, adenine nucleotide translocator and adenylate kinase causes mitochondrial energy deficit in human skin fibroblasts with chromosome 21 trisomy. Biochem J 431:299–310. doi:10.1042/BJ20100581
Valenti D, Manente GA, Moro L, Marra E, Vacca RA (2011) Deficit of complex I activity in human skin fibroblasts with chromosome 21 trisomy and overproduction of reactive oxygen species by mitochondria: involvement of the cAMP/PKA signalling pathway. Biochem J 435:679–688. doi:10.1042/BJ20101908
Bozzo F, Mirra A, Carrì MT (2017) Oxidative stress and mitochondrial damage in the pathogenesis of ALS: new perspectives. Neurosci Lett 636:3–8. doi:10.1016/j.neulet.2016.04.065
Carrì MT, D'Ambrosi N, Cozzolino M (2016) Pathways to mitochondrial dysfunction in ALS pathogenesis. Biochem Biophys Res Commun. doi:10.1016/j.bbrc.2016.07.055
Moreno-Ortega AJ, Al-Achbili LM, Alonso E, de Los Ríos C, García AG, Ruiz-Nuño A, Cano-Abad MF (2016) Neuroprotective effect of the novel compound ITH33/IQM9.21 against oxidative stress and Na(+) and Ca(2+) overload in motor neuron-like NSC-34 cells. Neurotox Res 30:380–391. doi:10.1007/s12640-016-9623-7
Zhou ZD, Saw WT, Tan EK (2016) Mitochondrial CHCHD-containing proteins: physiologic functions and link with neurodegenerative diseases. Mol Neurobiol. doi:10.1007/s12035-016-0099-5
Wang Q, Liu Y, Zou X, Wang Q, An M, Guan X, He J, Tong Y et al (2008) The hippocampal proteomic analysis of senescence-accelerated mouse: implications of Uchl3 and mitofilin in cognitive disorder and mitochondria dysfunction in SAMP8. Neurochem Res 33:1776–1782. doi:10.1007/s11064-008-9628-6
Eckert GP, Schiborr C, Hagl S, Abdel-Kader R, Müller WE, Rimbach G, Frank J (2013) Curcumin prevents mitochondrial dysfunction in the brain of the senescence-accelerated mouse-prone 8. Neurochem Int 62:595–602. doi:10.1016/j.neuint.2013.02.014
Wu FJ, Xue Y, Liu XF, Xue CH, Wang JF, Du L, Takahashi K, Wang YM (2014) The protective effect of eicosapentaenoic acid-enriched phospholipids from sea cucumber Cucumaria frondosa on oxidative stress in PC12 cells and SAMP8 mice. Neurochem Int 64:9–17. doi:10.1016/j.neuint.2013.10.015
Feng Y, Cui Y, Gao JL, Li R, Jiang XH, Tian YX, Wang KJ, Li MH et al (2016) Neuroprotective effects of resveratrol against traumatic brain injury in rats: involvement of synaptic proteins and neuronal autophagy. Mol Med Rep 13:5248–5254. doi:10.3892/mmr.2016.5201
Zhang Y, Cao X, Zhu W, Liu Z, Liu H, Zhou Y, Cao Y, Liu C et al (2016) Resveratrol enhances autophagic flux and promotes ox-LDL degradation in HUVECs via upregulation of SIRT1. Oxidative Med Cell Longev 2016:7589813. doi:10.1155/2016/7589813
Wu Y, Li X, Zhu JX, Xie W, Le W, Fan Z, Jankovic J, Pan T (2011) Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson's disease. Neurosignals 19:163–174. doi:10.1159/000328516
Lotharius J, Brundin P (2002) Pathogenesis of Parkinson's disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci 3:932–942
Olanow CW, Brundin P (2013) Parkinson's disease and alpha synuclein: is Parkinson's disease a prion-like disorder? Mov Disord 28:31–40. doi:10.1002/mds.25373
Zhang QS, Heng Y, Yuan YH, Chen NH (2016) Pathological α-synuclein exacerbates the progression of Parkinson's disease through microglial activation. Toxicol Lett 265:30–37. doi:10.1016/j.toxlet.2016.11.002
Mack JM, Schamne MG, Sampaio TB, Pértile RA, Fernandes PA, Markus RP, Prediger RD (2016) Melatoninergic system in Parkinson's disease: from neuroprotection to the management of motor and nonmotor symptoms. Oxidative Med Cell Longev 2016:3472032
Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK (2008) Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem 283:9089–9100. doi:10.1074/jbc.M710012200
Reeve AK, Park TK, Jaros E, Campbell GR, Lax NZ, Hepplewhite PD, Krishnan KJ, Elson JL et al (2012) Relationship between mitochondria and α-synuclein: a study of single substantia nigra neurons. Arch Neurol 69:385–393. doi:10.1001/archneurol.2011.2675
Di Maio R, Barrett PJ, Hoffman EK, Barrett CW, Zharikov A, Borah A, Hu X, McCoy J et al (2016) α-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson's disease. Sci Transl Med 8:342ra78. doi:10.1126/scitranslmed.aaf3634
Ingelsson M (2016) Alpha-synuclein oligomers-neurotoxic molecules in Parkinson's disease and other Lewy body disorders. Front Neurosci 10:408. doi:10.3389/fnins.2016.00408
Redmann M, Darley-Usmar V, Zhang J (2016) The role of autophagy, mitophagy and lysosomal functions in modulating bioenergetics and survival in the context of redox and Proteotoxic damage: implications for neurodegenerative diseases. Aging Dis 7:150–162
Jeong JK, Moon MH, Bae BC, Lee YJ, Seol JW, Kang HS, Kim JS, Kang SJ et al (2012) Autophagy induced by resveratrol prevents human prion protein-mediated neurotoxicity. Neurosci Res 73:99–105. doi:10.1016/j.neures.2012.03.005
Lin TK, Chen SD, Chuang YC, Lin HY, Huang CR, Chuang JH, Wang PW, Huang ST et al (2014) Resveratrol partially prevents rotenone-induced neurotoxicity in dopaminergic SH-SY5Y cells through induction of heme oxygenase-1 dependent autophagy. Int J Mol Sci 15:1625–1646. doi:10.3390/ijms15011625
Carchman EH, Rao J, Loughran PA, Rosengart MR, Zuckerbraun BS (2011) Heme oxygenase-1-mediated autophagy protects against hepatocyte cell death and hepatic injury from infection/sepsis in mice. Hepatology 53:2053–2062. doi:10.1002/hep.24324
Dong C, Zheng H, Huang S, You N, Xu J, Ye X, Zhu Q, Feng Y et al (2015) Heme oxygenase-1 enhances autophagy in podocytes as a protective mechanism against high glucose-induced apoptosis. Exp Cell Res 337:146–159. doi:10.1016/j.yexcr.2015.04.005
Surolia R, Karki S, Kim H, Yu Z, Kulkarni T, Mirov SB, Carter AB, Rowe SM et al (2015) Heme oxygenase-1-mediated autophagy protects against pulmonary endothelial cell death and development of emphysema in cadmium-treated mice. Am J Physiol Lung Cell Mol Physiol 309:L280–L292. doi:10.1152/ajplung.00097.2015
Belounis A, Nyalendo C, Le Gall R, Imbriglio TV, Mahma M, Teira P, Beaunoyer M, Cournoyer S et al (2016) Autophagy is associated with chemoresistance in neuroblastoma. BMC Cancer 16:891
Noonan J, Zarrer J, Murphy BM (2016) Targeting autophagy in glioblastoma. Crit Rev Oncog 21:241–252
Acknowledgements
None to declare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Jardim, F.R., de Rossi, F.T., Nascimento, M.X. et al. Resveratrol and Brain Mitochondria: a Review. Mol Neurobiol 55, 2085–2101 (2018). https://doi.org/10.1007/s12035-017-0448-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0448-z