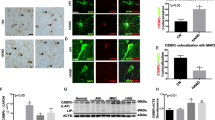
Abstract
Neurocognitive impairment (NCI) remains a significant cause of morbidity in human immunodeficiency virus (HIV)-positive individuals despite highly active antiretroviral therapy (HAART). White matter abnormalities have emerged as a key component of age-related neurodegeneration, and accumulating evidence suggests they play a role in HIV-associated neurocognitive disorders. Viral persistence in the brain induces chronic inflammation associated with lymphocytic infiltration, microglial proliferation, myelin loss, and cerebrovascular lesions. In this study, gene expression profiling was performed on frontal white matter from 34 older HIV+ individuals on HAART (18 with NCI) and 24 HIV-negative controls. We used the NanoString nCounter platform to evaluate 933 probes targeting inflammation, interferon and stress responses, energy metabolism, and central nervous system-related genes. Viral loads were measured using single-copy assays. Compared to HIV− controls, HIV+ individuals exhibited increased expression of genes related to interferon, MHC-1, and stress responses, myeloid cells, and T cells and decreased expression of genes associated with oligodendrocytes and energy metabolism in white matter. These findings correlated with increased white matter inflammation and myelin pallor, suggesting interferon (IRFs, IFITM1, ISG15, MX1, OAS3) and stress response (ATF4, XBP1, CHOP, CASP1, WARS) gene expression changes are associated with decreased energy metabolism (SREBF1, SREBF2, PARK2, TXNIP) and oligodendrocyte myelin production (MAG, MOG), leading to white matter dysfunction. Machine learning identified a 15-gene signature predictive of HIV status that was validated in an independent cohort. No specific gene expression patterns were associated with NCI. These findings suggest therapies that decrease chronic inflammation while protecting mitochondrial function may help to preserve white matter integrity in older HIV+ individuals.






Similar content being viewed by others
References
Sacktor N (2018) Changing clinical phenotypes of HIV-associated neurocognitive disorders. J Neurovirol 24(2):141–145. https://doi.org/10.1007/s13365-017-0556-6
Eggers C, Arendt G, Hahn K, Husstedt IW, Maschke M, Neuen-Jacob E, Obermann M, Rosenkranz T et al (2017) HIV-1-associated neurocognitive disorder: epidemiology, pathogenesis, diagnosis, and treatment. J Neurol 264(8):1715–1727. https://doi.org/10.1007/s00415-017-8503-2
Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A et al (2016) HIV-associated neurocognitive disorder--pathogenesis and prospects for treatment. Nat Rev Neurol 12(4):234–248. https://doi.org/10.1038/nrneurol.2016.27
Geffin R, McCarthy M (2018) Aging and apolipoprotein E in HIV infection. J Neurovirol 24(5):529–548. https://doi.org/10.1007/s13365-018-0660-2
Lamers SL, Rose R, Maidji E, Agsalda-Garcia M, Nolan DJ, Fogel GB, Salemi M, Garcia DL et al (2016) HIV DNA is frequently present within pathologic tissues evaluated at autopsy from combined antiretroviral therapy-treated patients with undetectable viral loads. J Virol 90(20):8968–8983. https://doi.org/10.1128/JVI.00674-16
Ko A, Kang G, Hattler JB, Galadima HI, Zhang J, Li Q, Kim WK (2019) Macrophages but not astrocytes harbor HIV DNA in the brains of HIV-1-infected aviremic individuals on suppressive antiretroviral therapy. J Neuroimmune Pharmacol 14(1):110–119. https://doi.org/10.1007/s11481-018-9809-2
Mackiewicz MM, Overk C, Achim CL, Masliah E (2019) Pathogenesis of age-related HIV neurodegeneration. J Neurovirol.:1–12. https://doi.org/10.1007/s13365-019-00728-z
Cysique LA, Brew BJ (2019) Vascular cognitive impairment and HIV-associated neurocognitive disorder: a new paradigm. J Neurovirol.:1–12. https://doi.org/10.1007/s13365-018-0706-5
Ulfhammer G, Eden A, Mellgren A, Fuchs D, Zetterberg H, Hagberg L, Nilsson S, Yilmaz A et al (2018) Persistent central nervous system immune activation following more than 10 years of effective HIV antiretroviral treatment. AIDS 32(15):2171–2178. https://doi.org/10.1097/QAD.0000000000001950
Cribbs DH, Berchtold NC, Perreau V, Coleman PD, Rogers J, Tenner AJ, Cotman CW (2012) Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J Neuroinflammation 9:179. https://doi.org/10.1186/1742-2094-9-179
Tavazzi E, Morrison D, Sullivan P, Morgello S, Fischer T (2014) Brain inflammation is a common feature of HIV-infected patients without HIV encephalitis or productive brain infection. Curr HIV Res 12(2):97–110
Raj D, Yin Z, Breur M, Doorduin J, Holtman IR, Olah M, Mantingh-Otter IJ, Van Dam D et al (2017) Increased white matter inflammation in aging- and Alzheimerʼs disease brain. Front Mol Neurosci 10:206. https://doi.org/10.3389/fnmol.2017.00206
Gelman BB, Chen T, Lisinicchia JG, Soukup VM, Carmical JR, Starkey JM, Masliah E, Commins DL et al (2012) The National NeuroAIDS Tissue Consortium brain gene array: two types of HIV-associated neurocognitive impairment. PloS one 7(9):e46178. https://doi.org/10.1371/journal.pone.0046178
Borjabad A, Morgello S, Chao W, Kim SY, Brooks AI, Murray J, Potash MJ, Volsky DJ (2011) Significant effects of antiretroviral therapy on global gene expression in brain tissues of patients with HIV-1-associated neurocognitive disorders. PLoS Pathog 7(9):e1002213. https://doi.org/10.1371/journal.ppat.1002213
Shah A, Gangwani MR, Chaudhari NS, Glazyrin A, Bhat HK, Kumar A (2016) Neurotoxicity in the post-HAART era: caution for the antiretroviral therapeutics. Neurotox Res 30(4):677–697. https://doi.org/10.1007/s12640-016-9646-0
Gullett JM, Lamb DG, Porges E, Woods AJ, Rieke J, Thompson P, Jahanshad N, Nir TM et al (2018) The impact of alcohol use on frontal white matter in HIV. Alcohol Clin Exp Res 42(9):1640–1649. https://doi.org/10.1111/acer.13823
Soontornniyomkij V, Umlauf A, Soontornniyomkij B, Gouaux B, Ellis RJ, Levine AJ, Moore DJ, Letendre SL (2018) Association of antiretroviral therapy with brain aging changes among HIV-infected adults. AIDS 32(14):2005–2015. https://doi.org/10.1097/QAD.0000000000001927
Su T, Wit FW, Caan MW, Schouten J, Prins M, Geurtsen GJ, Cole JH, Sharp DJ et al (2016) White matter hyperintensities in relation to cognition in HIV-infected men with sustained suppressed viral load on combination antiretroviral therapy. AIDS 30(15):2329–2339. https://doi.org/10.1097/QAD.0000000000001133
Hase Y, Horsburgh K, Ihara M, Kalaria RN (2018) White matter degeneration in vascular and other ageing-related dementias. J Neurochem 144(5):617–633. https://doi.org/10.1111/jnc.14271
Sanford R, Strain J, Dadar M, Maranzano J, Bonnet A, Mayo NE, Scott SC, Fellows LK et al (2019) HIV infection and cerebral small vessel disease are independently associated with brain atrophy and cognitive impairment. AIDS 33(7):1197–1205. https://doi.org/10.1097/QAD.0000000000002193
Underwood J, Cole JH, Caan M, De Francesco D, Leech R, van Zoest RA, Su T, Geurtsen GJ et al (2017) Gray and white matter abnormalities in treated human immunodeficiency virus disease and their relationship to cognitive function. Clin Infect Dis 65(3):422–432. https://doi.org/10.1093/cid/cix301
Kuhn T, Kaufmann T, Doan NT, Westlye LT, Jones J, Nunez RA, Bookheimer SY, Singer EJ et al (2018) An augmented aging process in brain white matter in HIV. Hum Brain Mapp 39(6):2532–2540. https://doi.org/10.1002/hbm.24019
Everall I, Vaida F, Khanlou N, Lazzaretto D, Achim C, Letendre S, Moore D, Ellis R et al (2009) Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J Neurovirol 15(5-6):360–370. https://doi.org/10.3109/13550280903131915
Solomon IH, De Girolami U, Chettimada S, Misra V, Singer EJ, Gabuzda D (2017) Brain and liver pathology, amyloid deposition, and interferon responses among older HIV-positive patients in the late HAART era. BMC Infect Dis 17(1):151. https://doi.org/10.1186/s12879-017-2246-7
Gill AJ, Kovacsics CE, Cross SA, Vance PJ, Kolson LL, Jordan-Sciutto KL, Gelman BB, Kolson DL (2014) Heme oxygenase-1 deficiency accompanies neuropathogenesis of HIV-associated neurocognitive disorders. J Clin Invest 124(10):4459–4472. https://doi.org/10.1172/JCI72279
Oka S, Leon J, Sakumi K, Ide T, Kang D, LaFerla FM, Nakabeppu Y (2016) Human mitochondrial transcriptional factor A breaks the mitochondria-mediated vicious cycle in Alzheimerʼs disease. Sci Rep 6:37889. https://doi.org/10.1038/srep37889
Nooka S, Ghorpade A (2018) Organellar stress intersects the astrocyte endoplasmic reticulum, mitochondria and nucleolus in HIV associated neurodegeneration. Cell Death Dis 9(3):317. https://doi.org/10.1038/s41419-018-0341-3
Morgello S, Gelman BB, Kozlowski PB, Vinters HV, Masliah E, Cornford M, Cavert W, Marra C et al (2001) The National NeuroAIDS Tissue Consortium: a new paradigm in brain banking with an emphasis on infectious disease. Neuropathol Appl Neurobiol 27(4):326–335
Somsouk M, Dunham RM, Cohen M, Albright R, Abdel-Mohsen M, Liegler T, Lifson J, Piatak M et al (2014) The immunologic effects of mesalamine in treated HIV-infected individuals with incomplete CD4+ T cell recovery: a randomized crossover trial. PloS one 9(12):e116306. https://doi.org/10.1371/journal.pone.0116306
Thomas JA, Gagliardi TD, Alvord WG, Lubomirski M, Bosche WJ, Gorelick RJ (2006) Human immunodeficiency virus type 1 nucleocapsid zinc-finger mutations cause defects in reverse transcription and integration. Virology 353(1):41–51. https://doi.org/10.1016/j.virol.2006.05.014
Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, Fell HP, Ferree S et al (2008) Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol 26(3):317–325. https://doi.org/10.1038/nbt1385
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK (2015) Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43(7):e47. https://doi.org/10.1093/nar/gkv007
Troyanskaya O, Cantor M, Sherlock G, Brown P, Hastie T, Tibshirani R, Botstein D, Altman RB (2001) Missing value estimation methods for DNA microarrays. Bioinformatics 17(6):520–525
Scagnolari C, Monteleone K, Selvaggi C, Pierangeli A, D'Ettorre G, Mezzaroma I, Turriziani O, Gentile M et al (2016) ISG15 expression correlates with HIV-1 viral load and with factors regulating T cell response. Immunobiology 221(2):282–290. https://doi.org/10.1016/j.imbio.2015.10.007
Schneider WM, Chevillotte MD, Rice CM (2014) Interferon-stimulated genes: a complex web of host defenses. Annu Rev Immunol 32:513–545. https://doi.org/10.1146/annurev-immunol-032713-120231
Fagone P, Nunnari G, Lazzara F, Longo A, Cambria D, Distefano G, Palumbo M, Nicoletti F et al (2016) Induction of OAS gene family in HIV monocyte infected patients with high and low viral load. Antiviral Res 131:66–73. https://doi.org/10.1016/j.antiviral.2016.04.009
Rosebeck S, Leaman DW (2008) Mitochondrial localization and pro-apoptotic effects of the interferon-inducible protein ISG12a. Apoptosis 13(4):562–572. https://doi.org/10.1007/s10495-008-0190-0
Camargo N, Goudriaan A, van Deijk AF, Otte WM, Brouwers JF, Lodder H, Gutmann DH, Nave KA et al (2017) Oligodendroglial myelination requires astrocyte-derived lipids. PLoS Biol 15(5):e1002605. https://doi.org/10.1371/journal.pbio.1002605
Voskuhl RR, Itoh N, Tassoni A, Matsukawa MA, Ren E, Tse V, Jang E, Suen TT et al (2019) Gene expression in oligodendrocytes during remyelination reveals cholesterol homeostasis as a therapeutic target in multiple sclerosis. Proc Natl Acad Sci U S A 116(20):10130–10139. https://doi.org/10.1073/pnas.1821306116
Wendelken LA, Jahanshad N, Rosen HJ, Busovaca E, Allen I, Coppola G, Adams C, Rankin KP et al (2016) ApoE epsilon4 is associated with cognition, brain integrity, and atrophy in HIV over age 60. J Acquir Immune Defic Syndr 73(4):426–432. https://doi.org/10.1097/QAI.0000000000001091
Shimano H (2002) Sterol regulatory element-binding protein family as global regulators of lipid synthetic genes in energy metabolism. Vitam Horm 65:167–194
Siangphoe U, Archer KJ (2015) Gene expression in HIV-associated neurocognitive disorders: a meta-analysis. J Acquir Immune Defic Syndr 70(5):479–488. https://doi.org/10.1097/QAI.0000000000000800
Sagar V, Pilakka-Kanthikeel S, Martinez PC, Atluri VSR, Nair M (2017) Common gene-network signature of different neurological disorders and their potential implications to neuroAIDS. PLoS One 12(8):e0181642. https://doi.org/10.1371/journal.pone.0181642
Furman D, Chang J, Lartigue L, Bolen CR, Haddad F, Gaudilliere B, Ganio EA, Fragiadakis GK et al (2017) Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat Med 23(2):174–184. https://doi.org/10.1038/nm.4267
Nissen SK, Hojen JF, Andersen KL, Kofod-Olsen E, Berg RK, Paludan SR, Ostergaard L, Jakobsen MR et al (2014) Innate DNA sensing is impaired in HIV patients and IFI16 expression correlates with chronic immune activation. Clin Exp Immunol 177(1):295–309. https://doi.org/10.1111/cei.12317
Thompson MR, Sharma S, Atianand M, Jensen SB, Carpenter S, Knipe DM, Fitzgerald KA, Kurt-Jones EA (2014) Interferon gamma-inducible protein (IFI) 16 transcriptionally regulates type i interferons and other interferon-stimulated genes and controls the interferon response to both DNA and RNA viruses. J Biol Chem 289(34):23568–23581. https://doi.org/10.1074/jbc.M114.554147
Jonsson KL, Laustsen A, Krapp C, Skipper KA, Thavachelvam K, Hotter D, Egedal JH, Kjolby M et al (2017) IFI16 is required for DNA sensing in human macrophages by promoting production and function of cGAMP. Nat Commun 8:14391. https://doi.org/10.1038/ncomms14391
Sanfilippo C, Pinzone MR, Cambria D, Longo A, Palumbo M, Di Marco R, Condorelli F, Nunnari G et al (2018) OAS gene family expression is associated with HIV-related neurocognitive disorders. Molecular neurobiology 55(3):1905–1914. https://doi.org/10.1007/s12035-017-0460-3
Cotto B, Natarajaseenivasan K, Langford D (2019) HIV-1 infection alters energy metabolism in the brain: contributions to HIV-associated neurocognitive disorders. Prog Neurobiol 101616. https://doi.org/10.1016/j.pneurobio.2019.101616
Codoni V, Blum Y, Civelek M, Proust C, Franzen O, Cardiogenics C, CADGenomics ILC, Bjorkegren JL et al (2016) Preservation analysis of macrophage gene coexpression between human and mouse identifies PARK2 as a genetically controlled master regulator of oxidative phosphorylation in humans. G3 (Bethesda) 6(10):3361–3371. https://doi.org/10.1534/g3.116.033894
Gupta A, Anjomani-Virmouni S, Koundouros N, Dimitriadi M, Choo-Wing R, Valle A, Zheng Y, Chiu YH et al (2017) PARK2 depletion connects energy and oxidative stress to PI3K/Akt activation via PTEN S-nitrosylation. Mol Cell 65(6):999–1013 e1017. https://doi.org/10.1016/j.molcel.2017.02.019
Witte ME, Bol JG, Gerritsen WH, van der Valk P, Drukarch B, van Horssen J, Wilhelmus MM (2009) Parkinsonʼs disease-associated parkin colocalizes with Alzheimerʼs disease and multiple sclerosis brain lesions. Neurobiol Dis 36(3):445–452. https://doi.org/10.1016/j.nbd.2009.08.009
Kang I, Chu CT, Kaufman BA (2018) The mitochondrial transcription factor TFAM in neurodegeneration: emerging evidence and mechanisms. FEBS Lett 592(5):793–811. https://doi.org/10.1002/1873-3468.12989
Nasoohi S, Ismael S, Ishrat T (2018) Thioredoxin-interacting protein (TXNIP) in cerebrovascular and neurodegenerative diseases: regulation and implication. Mol Neurobiol 55(10):7900–7920. https://doi.org/10.1007/s12035-018-0917-z
Bandera A, Masetti M, Fabbiani M, Biasin M, Muscatello A, Squillace N, Clerici M, Gori A et al (2018) The NLRP3 inflammasome is upregulated in HIV-infected antiretroviral therapy-treated individuals with defective immune recovery. Front Immunol 9:214. https://doi.org/10.3389/fimmu.2018.00214
Shimano H, Sato R (2017) SREBP-regulated lipid metabolism: convergent physiology - divergent pathophysiology. Nat Rev Endocrinol 13(12):710–730. https://doi.org/10.1038/nrendo.2017.91
Pitale PM, Gorbatyuk O, Gorbatyuk M (2017) Neurodegeneration: keeping ATF4 on a tight leash. Front Cell Neurosci 11:410. https://doi.org/10.3389/fncel.2017.00410
Kearns AC, Robinson JA, Shekarabi M, Liu F, Qin X, Burdo TH (2018) Caspase-1-associated immune activation in an accelerated SIV-infected rhesus macaque model. J Neurovirol 24(4):420–431. https://doi.org/10.1007/s13365-018-0630-8
Dillman AA, Majounie E, Ding J, Gibbs JR, Hernandez D, Arepalli S, Traynor BJ, Singleton AB et al (2017) Transcriptomic profiling of the human brain reveals that altered synaptic gene expression is associated with chronological aging. Sci Rep 7(1):16890–16812. https://doi.org/10.1038/s41598-017-17322-0
Darmanis S, Sloan SA, Zhang Y, Enge M, Caneda C, Shuer LM, Hayden Gephart MG, Barres BA et al (2015) A survey of human brain transcriptome diversity at the single cell level. Proc Natl Acad Sci U S A 112(23):7285–7290. https://doi.org/10.1073/pnas.1507125112
Acknowledgments
We thank the Dana-Farber/Harvard Cancer Center Molecular Biology Core for the NanoString nCounter platform services. This work was supported by National Institutes of Health grants to D.G. (R01 MH097659 and R01 DA40391). Financial support for the NNTC was provided through the following cooperative agreements from the National Institutes of Health: U24MH100930, U24MH100931, U24MH100928, U24MH100929, and U24MH100925. This study was supported in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. HSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of any trade names, commercial products, or organizations imply endorsement by the US government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(XLSX 80 kb)
Rights and permissions
About this article
Cite this article
Solomon, I.H., Chettimada, S., Misra, V. et al. White Matter Abnormalities Linked to Interferon, Stress Response, and Energy Metabolism Gene Expression Changes in Older HIV-Positive Patients on Antiretroviral Therapy. Mol Neurobiol 57, 1115–1130 (2020). https://doi.org/10.1007/s12035-019-01795-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01795-3