Abstract
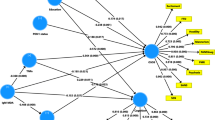
There is now evidence that schizophrenia and deficit schizophrenia are neuro-immune conditions and that oxidative stress toxicity (OSTOX) may play a pathophysiological role. Aims of the study: to compare OSTOX biomarkers and antioxidant (ANTIOX) defenses in deficit versus non-deficit schizophrenia. We examined lipid hydroperoxides (LOOH), malondialdehyde (MDA), advanced oxidation protein products (AOPP), sulfhydryl (–SH) groups, paraoxonase 1 (PON1) activity and PON1 Q192R genotypes, and total radical-trapping antioxidant parameter (TRAP) as well as immune biomarkers in patients with deficit (n = 40) and non-deficit (n = 40) schizophrenia and healthy controls (n = 40). Deficit schizophrenia is characterized by significantly increased levels of AOPP and lowered –SH, and PON1 activity, while no changes in the OSTOX/ANTIOX biomarkers were found in non-deficit schizophrenia. An increased OSTOX/ANTIOX ratio was significantly associated with deficit versus non-deficit schizophrenia (odds ratio = 3.15, p < 0.001). Partial least squares analysis showed that 47.6% of the variance in a latent vector extracted from psychosis, excitation, hostility, mannerism, negative symptoms, psychomotor retardation, formal thought disorders, and neurocognitive test scores was explained by LOOH+AOPP, PON1 genotype + activity, CCL11, tumor necrosis factor (TNF)-α, and IgA responses to neurotoxic tryptophan catabolites (TRYCATs), whereas –SH groups and IgM responses to MDA showed indirect effects mediated by OSTOX and neuro-immune biomarkers. When overall severity of schizophrenia increases, multiple immune and oxidative (especially protein oxidation indicating chlorinative stress) neurotoxicities and impairments in immune-protective resilience become more prominent and shape a distinct nosological entity, namely deficit schizophrenia. The nomothetic network psychiatry approach allows building causal-pathway-phenotype models using machine learning techniques.






Similar content being viewed by others
Abbreviations
- Th:
-
T helper
- TRYCATs:
-
Tryptophan catabolites
- IRS:
-
Immune-inflammatory response system
- CIRS:
-
Compensatory immune regulatory system
- IL:
-
Interleukin
- sIL-1RA:
-
Soluble IL-1 receptor antagonist
- TNF:
-
Tumor necrosis factor
- PHEMN:
-
Psychosis, hostility, excitation, mannerism, negative symptoms
- PA:
-
Picolinic acid
- XA:
-
Xanthurenic acid
- OSE:
-
Oxidative-specific epitopes
- MDA:
-
Malondialdehyde
- PON:
-
Paraoxonase
- O&NS:
-
Oxidative and nitrosative stress
- LOOH:
-
Lipid hydroperoxides
- NOx:
-
Nitric oxide metabolites
- TRAP:
-
Radical-trapping antioxidant parameter
- AOPP:
-
Advanced oxidation protein products
- –SH:
-
Thiol groups
- SDS:
-
Schedule for Deficit Schizophrenia
- PANSS:
-
Positive and Negative Syndrome Scale
- SANS:
-
Scale for the Assessment of Negative Symptoms
- PMR:
-
Psychomotor retardation
- FTD:
-
Formal thought disorders
- CERAD:
-
Consortium to Establish a Registry for Alzheimer’s Disease
- CANTAB:
-
Cambridge Neuropsychological Test Automated Battery
- WLM:
-
World List Memory test
- VFT:
-
Verbal Fluency Test
- MMSE:
-
Mini-Mental State Examination
- SWM:
-
Spatial working memory
- TUD:
-
Tobacco use disorder
- BMI:
-
Body mass index
- CMPA:
-
4-(Chloromethyl)phenyl acetate
- AREase:
-
Arylesterase
- 3OSTOX:
-
Oxidative stress toxicity index
- 3ANTIOX:
-
Antioxidant index
- 3OHK:
-
3-OH-kynurenine
- AA:
-
Anthranilic acid
- KA:
-
Kynurenic acid
- NOX/PRO:
-
Noxious/protective ratio
- 4PRORESIL:
-
Index of protective resilience against neuro-immune, neuro-oxidative, and bacterial stressors
- 8MITOTOX:
-
Index of multiple immune and oxidative toxicities
- GLM:
-
Generalized linear model
- FDR:
-
False discovery rate
- PLS:
-
Partial least squares
- AVE:
-
Average extracted variance
- LV:
-
Latent vector
- OSOS:
-
Overall severity of schizophrenia
- CTD:
-
Confirmatory tetrad analysis
- NNP:
-
Nomothetic network psychiatry
References
Smith RS, Maes M (1995) The macrophage-T-lymphocyte theory of schizophrenia: additional evidence. Med Hypotheses 45(2):135–141
Maes M, Delange J, Ranjan R, Meltzer HY, Desnyder R, Cooremans W, Scharpé S (1997) Acute phase proteins in schizophrenia, mania and major depression: modulation by psychotropic drugs. Psychiatry Res 66(1):1–11
Roomruangwong C, Noto C, Kanchanatawan B, Anderson G, Kubera M, Carvalho AF, Maes M (2020) The role of aberrations in the immune-inflammatory response system (IRS) and the compensatory immune-regulatory reflex system (CIRS) in different phenotypes of schizophrenia: the IRS-CIRS theory of schizophrenia. Mol Neurobiol 57(2):778–797
Maes M, Vojdani A, Geffard M, Moreira EG, Barbosa DS, Michelin AP, Semeão LO, Sirivichayakul S et al (2019) Schizophrenia phenomenology comprises a bifactorial general severity and a single-group factor, which are differently associated with neurotoxic immune and immune-regulatory pathways. Biomol Concepts 10(1):209–225
Maes M, Sirivichayakul S, Kanchanatawan B, Carvalho AF (2020) In schizophrenia, psychomotor retardation is associated with executive and memory impairments, negative and psychotic symptoms, neurotoxic immune products and lower natural IgM to malondialdehyde. World J Biol Psychiatry 7:1–19
Al-Hakeim HK, Almulla AF, Maes M (2020) The neuroimmune and neurotoxic fingerprint of major neurocognitive psychosis or deficit schizophrenia: a supervised machine learning study. Neurotox Res 37(3):753–771
Kanchanatawan B, Sirivichayakul S, Ruxrungtham K, Carvalho AF, Geffard M, Ormstad H, Anderson G, Maes M (2018) Deficit, but not nondeficit, schizophrenia is characterized by mucosa-associated activation of the tryptophan catabolite (TRYCAT) pathway with highly specific increases in IgA responses directed to picolinic, xanthurenic, and quinolinic acid. Mol Neurobiol 55(2):1524–1536
Kanchanatawan B, Sriswasdi S, Thika S, Stoyanov D, Sirivichayakul S, Carvalho AF, Geffard M, Maes M (2018) Towards a new classification of stable phase schizophrenia into major and simple neuro-cognitive psychosis: results of unsupervised machine learning analysis. J Eval Clin Pract 24(4):879–891
Sirivichayakul S, Kanchanatawan B, Thika S, Carvalho AF, Maes M (2019) Eotaxin, an endogenous cognitive deteriorating chemokine (ECDC), is a major contributor to cognitive decline in normal people and to executive, memory, and sustained attention deficits, formal thought disorders, and psychopathology in schizophrenia patients. Neurotox Res 35(1):122–138
Maes M, Kanchanatawan B, Sirivichayakul S, Carvalho AF (2019) In schizophrenia, deficits in natural IgM isotype antibodies including those directed to malondialdehyde and azelaic acid strongly predict negative symptoms, neurocognitive impairments, and the deficit syndrome. Mol Neurobiol 56(7):5122–5135
Maes M, Sirivichayakul S, Matsumoto AK, Maes A, Michelin AP, de Oliveira SL, de Lima Pedrão JV, Moreira EG et al (2020) Increased levels of plasma tumor necrosis factor-α mediate schizophrenia symptom dimensions and neurocognitive impairments and are inversely associated with natural IgM directed to malondialdehyde and paraoxonase 1 activity. Mol Neurobiol 57(5):2333–2345
Maes M, Sirivichayakul S, Kanchanatawan B, Vodjani A (2019) Breakdown of the paracellular tight and adherens junctions in the gut and blood brain barrier and damage to the vascular barrier in patients with deficit schizophrenia. Neurotox Res 36(2):306–322
Maes M, Kanchanatawan B, Sirivichayakul S, Carvalho AF (2019) In schizophrenia, increased plasma IgM/IgA responses to gut commensal bacteria are associated with negative symptoms, neurocognitive impairments, and the deficit phenotype. Neurotox Res 35(3):684–698
Morris G, Puri BK, Olive L, Carvalho AF, Berk M, Maes M (2019) Emerging role of innate B1 cells in the pathophysiology of autoimmune and neuroimmune diseases: association with inflammation, oxidative and nitrosative stress and autoimmune responses. Pharmacol Res 48:104408
Kanchanatawan B, Sirivichayakul S, Ruxrungtham K, Carvalho AF, Geffard M, Anderson G, Maes M (2018) Deficit schizophrenia is characterized by defects in IgM-mediated responses to tryptophan catabolites (TRYCATs): a paradigm shift towards defects in natural self-regulatory immune responses coupled with mucosa-derived TRYCAT pathway activation. Mol Neurobiol 55(3):2214–2226
Matsumoto AK, Maes M, Supasitthumrong T, Maes A, Michelin AP, de Oliveira SL, de Lima Pedrão JV, Moreira EG et al (2020) Deficit schizophrenia and its features are associated with PON1 Q192R genotypes and lowered paraoxonase 1 (PON1) enzymatic activity: effects on bacterial translocation. CNS Spectrums in press
Moylan S, Berk M, Dean OM, Samuni Y, Williams LJ, O'Neil A, Hayley AC, Pasco JA et al (2014) Oxidative & nitrosative stress in depression: why so much stress? Neurosci Biobehav Rev 45:46–62
Noto C, Ota VK, Gadelha A, Noto MN, Barbosa DS, Bonifácio KL, Nunes SO, Cordeiro Q et al (2015) Oxidative stress in drug naïve first episode psychosis and antioxidant effects of risperidone. J Psychiatr Res 68:210–216
Sarandol A, Sarandol E, Acikgoz HE, Eker SS, Akkaya C, Dirican M (2015) First-episode psychosis is associated with oxidative stress: effects of short-term antipsychotic treatment. Psychiatry Clin Neurosci 69(11):699–707
García-Bueno B, Bioque M, Mac-Dowell KS, Barcones MF, Martínez-Cengotitabengoa M, Pina-Camacho L, Rodríguez-Jiménez R, Sáiz PA et al (2014) Pro−/anti-inflammatory dysregulation in patients with first episode of psychosis: toward an integrative inflammatory hypothesis of schizophrenia. Schizophr Bull 40(2):376–387
Pedrini M, Massuda R, Fries GR, de Bittencourt Pasquali MA, Schnorr CE, Moreira JC, Teixeira AL, Lobato MI et al (2012) Similarities in serum oxidative stress markers and inflammatory cytokines in patients with overt schizophrenia at early and late stages of chronicity. J Psychiatr Res 46(6):819–824
Kriisa K, Haring L, Vasar E, Koido K, Janno S, Vasar V, Zilmer K, Zilmer M (2016) Antipsychotic treatment reduces indices of oxidative stress in first-episode psychosis patients. Oxidative Med Cell Longev 2016:9616593
Boll KM, Noto C, Bonifácio KL, Bortolasci CC, Gadelha A, Bressan RA, Barbosa DS, Maes M et al (2017) Oxidative and nitrosative stress biomarkers in chronic schizophrenia. Psychiatry Res 253:43–48
Tsugawa S, Noda Y, Tarumi R, Mimura Y, Yoshida K, Iwata Y, Elsalhy M, Kuromiya M et al (2019) Glutathione levels and activities of glutathione metabolism enzymes in patients with schizophrenia: a systematic review and meta-analysis. J Psychopharmacol 33(10):1199–1214
Fraguas D, Díaz-Caneja CM, Rodríguez-Quiroga A, Arango C (2017) Oxidative stress and inflammation in early onset first episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol 20(6):435–444
Fraguas D, Díaz-Caneja CM, Ayora M, Hernández-Álvarez F, Rodríguez-Quiroga A, Recio S, Leza JC, Arango C (2019) Oxidative stress and inflammation in first-episode psychosis: a systematic review and meta-analysis. Schizophr Bull 45(4):742–751
Das TK, Javadzadeh A, Dey A, Sabesan P, Théberge J, Radua J, Palaniyappan L (2019) Antioxidant defense in schizophrenia and bipolar disorder: a meta-analysis of MRS studies of anterior cingulate glutathione. Prog Neuro-Psychopharmacol Biol Psychiatry 91:94–102
Zhang M, Zhao Z, He L, Wan C (2010) A meta-analysis of oxidative stress markers in schizophrenia. Sci China Life Sci 53(1):112–124
Grignon S, Chianetta JM (2007) Assessment of malondialdehyde levels in schizophrenia: a meta-analysis and some methodological considerations. Prog Neuro-Psychopharmacol Biol Psychiatry 31(2):365–369
Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT Jr (1989) The schedule for the deficit syndrome: an instrument for research in schizophrenia. Psychiatry Res 30:119–123
Kittirathanapaiboon P, Khamwongpin M (2005) The validity of the mini international neuropsychiatric interview (M.I.N.I.) Thai version: Suanprung hospital, Department of Mental Health.
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276
Andreasen NC (1989) The scale for the assessment of negative symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl 7:49–58
Overall JE, Gorham DR (1962) The brief psychiatric rating scale. Psychol Rep 10:799–812
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62
Almulla AF, Al-Hakeim H, Maes M (2020) Schizophrenia phenomenology revisited: positive and negative symptoms are strongly related reflective manifestations of an underlying single trait indicating overall severity of schizophrenia. CNS Spectrums, in press:1–10
CERAD (1986) CERAD – an overview: the consortium to establish a registry for Alzheimer’s disease; http://cerad.mc.duke.edu/
CANTAB (2018) The most validated cognitive research software.http://www.cambridgecognition.com/cantab/ October 1, 2018
Hanasand M, Omdal R, Norheim KB, Gransson LG, Brede C, Jonsson G (2012) Improved detection of advanced oxidation protein products in plasma. Clin Chim Acta 413:901–906
Gonzalez Flecha B, Llesuy S, Boveris A (1991) Hydroperoxide-initiated chemiluminescence: an assay for oxidative stress in biopsies of heart, liver, and muscle. Free Radic Biol Med 10:93–100
Panis C, Herrera ACSA, Victorino VJ, Campos FC, Freitas LF, De Rossi T, Colado Simao AN, Cecchini AL et al (2012) Oxidative stress and hematological profiles of advanced breast cancer patients subjected to paclitaxel or doxorubicin chemotherapy. Breast Cancer Res Treat 133:89–97
Navarro-Gonzalvez JA, Garcia-Benayas C, Arenas J (1998) Semiautomated measurement of nitrate in biological fluids. Clin Chem 44:679–681
Repetto M, Reides C, Carretero MLG, Costa M, Griemberg G, Llesuy S (1996) Oxidative stress in blood of HIV infected patients. Clin Chim Acta 255(2):107–117
Hu ML (1994) Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol 233:380–385
Taylan E, Resmi H (2010) The analytical performance of a microplatemethod for total sulfhydryl measurement in biological samples. Turkish J Biochem 35:275–278
Richter RJ, Jarvik GP, Furlong CE (2008) Determination of paraoxonase 1 status without the use of toxic organophosphate substrates. Circ Cardiovasc Genet 1:147–152
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistics Society Series b (Methodological) 57:289–300
Ringle CM, da Silva D, Bido D (2014) Structural equation modeling with the SmartPLS. Brazilian J Market- BJM Revista Brasileira de Marketing – ReMark Edição Especial Vol 13, n. 2.
Albayrak Y, Ünsal C, Beyazyüz M, Ünal A, Kuloğlu M (2013) Reduced total antioxidant level and increased oxidative stress in patients with deficit schizophrenia: a preliminary study. Prog Neuro-Psychopharmacol Biol Psychiatry 45:144–149
Wu Q, Zhong ZM, Pan Y, Zeng JH, Zheng S, Zhu SY, Chen JT (2015) Advanced oxidation protein products as a novel marker of oxidative stress in postmenopausal osteoporosis. Med Sci Monit 21:2428–2432
Yap YW, Whiteman M, Cheung NS (2007) Chlorinative stress: an under appreciated mediator of neurodegeneration? Cell Signal 19(2):219–228
Casciaro M, Di Salvo E, Pace E, Ventura-Spagnolo E, Navarra M, Gangemi S (2017) Chlorinative stress in age-related diseases: a literature review. Immun Ageing 14:21
Rasool M, Malik A, Butt TT, Ashraf MAB, Rasool R, Zahid A, Waquar S, Asif M et al (2019) Implications of advanced oxidation protein products (AOPPs), advanced glycation end products (AGEs) and other biomarkers in the development of cardiovascular diseases. Saudi J Biol Sci 26(2):334–339
Yu C, Huang D, Wang K, Lin B, Liu Y, Liu S, Wu W, Zhang H (2017) Advanced oxidation protein products induce apoptosis, and upregulate sclerostin and RANKL expression, in osteocytic MLO-Y4 cells via JNK/p38 MAPK activation. Mol Med Rep 15(2):543–550
Estévez M, Luna C (2017) Dietary protein oxidation: a silent threat to human health? Crit Rev Food Sci Nutr 57(17):3781–3793
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97(6):1634–1658
Ding R, Jiang H, Sun B, Wu X, Li W, Zhu S, Liao C, Zhong Z et al (2016) Advanced oxidation protein products sensitized the transient receptor potential vanilloid 1 via NADPH oxidase 1 and 4 to cause mechanical hyperalgesia. Redox Biol 10:1–11
de Carvalho Jennings Pereira WL, Flauzino T, Alfieri DF, Oliveira SR, Kallaur AP, Simão ANC, Lozovoy MAB, Kaimen-Maciel DR et al (2020) Immune-inflammatory, metabolic and hormonal biomarkers are associated with the clinical forms and disability progression in patients with multiple sclerosis: a follow-up study. J Neurol Sci 410:116630
Scavuzzi BM, Simão ANC, Iriyoda TMV, Lozovoy MAB, Stadtlober NP, Franchi Santos LFDR, Flauzino T, de Medeiros FA et al (2018) Increased lipid and protein oxidation and lowered anti-oxidant defenses in systemic lupus erythematosus are associated with severity of illness, autoimmunity, increased adhesion molecules, and Th1 and Th17 immune shift. Immunol Res 66(1):158–171
Medeiros MS, Schumacher-Schuh A, Cardoso AM, Bochi GV, Baldissarelli J, Kegler A, Santana D, Chaves CM et al (2016) Iron and oxidative stress in Parkinson’s disease: an observational study of injury biomarkers. PLoS One 11(1):e0146129
Maes M, Supasitthumrong T, Limotai C, Michelin AP, Matsumoto AK, Semeão LDO, Pedrão JVDL, Moreira EG et al (2020) Increased oxidative stress toxicity and lowered antioxidant defenses in temporal lobe epilepsy and mesial temporal sclerosis: associations with psychiatric comorbidities. Preprints:2020010285. https://doi.org/10.20944/preprints202001.0285.v1
Maes M, Bonifacio KL, Morelli NR, Vargas HO, Moreira EG, St Stoyanov D, Barbosa DS, Carvalho AF et al (2018) Generalized anxiety disorder (GAD) and comorbid major depression with GAD are characterized by enhanced nitro-oxidative stress, increased lipid peroxidation, and lowered lipid-associated antioxidant defenses. Neurotox Res 34(3):489–510
Gomes C, Martinho FC, Barbosa DS, Antunes LS, Póvoa HCC, Baltus THL, Morelli NR, Vargas HO et al (2018) Increased root canal endotoxin levels are associated with chronic apical periodontitis, increased oxidative and nitrosative stress, major depression, severity of depression, and a lowered quality of life. Mol Neurobiol 55(4):2814–2827
Roomruangwong C, Barbosa DS, Matsumoto AK, Nogueira AS, Kanchanatawan B, Sirivichayakul S, Carvalho AF, Duleu S et al (2017) Activated neuro-oxidative and neuro-nitrosative pathways at the end of term are associated with inflammation and physio-somatic and depression symptoms, while predicting outcome characteristics in mother and baby. J Affect Disord 223:49–58
Zhang P, Wang H, Hong Y, Yu M, Zeng R, Long Y, Chen J (2018) Selective visualization of endogenous hypochlorous acid in zebrafish during lipopolysaccharide-induced acute liver injury using a polymer micelles-based ratiometric fluorescent probe. Biosens Bioelectron 99:318–324
Thomas CJ, Schroder K (2013) Pattern recognition receptor function in neutrophils. Trends Immunol 34(7):317–328
Oxford Biomedical Research (2010) Antioxidants and their measurement. Antioxidant assays: how do they compare, As accessed March 3, 2020 https://www.oxfordbiomed.com/tech-resources/oxidative-stress-best-practices/antioxidants-and-their-measurement
Rahman I, MacNee W (2000) Regulation of redox glutathione levels and gene transcription in lung inflammation: therapeutic approaches. Free Radic Biol Med 28(9):1405–1420
Biswas S, Chida AS, Rahman I (2006) Redox modifications of protein-thiols: emerging roles in cell signaling. Biochem Pharmacol 71(5):551–564
Wei C, Sun Y, Chen N, Chen S, Xiu M, Zhang X (2020) Interaction of oxidative stress and BDNF on executive dysfunction in patients with chronic schizophrenia. Psychoneuroendocrinology 111:104473
Bai ZL, Li XS, Chen GY, Du Y, Wei ZX, Chen X, Zheng GE, Deng W et al (2018) Serum oxidative stress marker levels in unmedicated and medicated patients with schizophrenia. J Mol Neurosci 66(3):428–436
Moreira EG, Boll KM, Correia DG, Soares JF, Rigobello C, Maes M (2019) Why should psychiatrists and neuroscientists worry about paraoxonase 1? Curr Neuropharmacol 7(11):1004–1020
Gugliucci A, Menini T (2015) Paraoxonase 1 and HDL maturation. Clin Chim Acta 439:5–13
Huang Y, Wu Z, Riwanto M, Gao S, Levison BS, Gu X, Fu X, Wagner MA et al (2013) Myeloperoxidase, paraoxonase-1, and HDL form a functional ternary complex. J Clin Invest 123(9):3815–3828
Efrat M, Aviram M (2010) Paraoxonase 1 interactions with HDL, antioxidants and macrophages regulate atherogenesis - a protective role for HDL phospholipids. Adv Exp Med Biol 660:153–166
Brinholi FF, Noto C, Maes M, Bonifácio KL, Brietzke E, Ota VK, Gadelha A, Cordeiro Q, Belangero SI, Bressan RA, Vargas HO, Higachi L, de FariasCC Moreira EG, Barbosa DS (2015) Lowered paraoxonase 1 (PON1) activity is associated with increased cytokine levels in drug naïve first episode psychosis. Schizophr Res 166(1–3):225–230. https://doi.org/10.1016/j.schres.2015.06.009
Meneses MJ, Silvestre R, Sousa-Lima I, Macedo MP (2019) Paraoxonase-1 as a regulator of glucose and lipid homeostasis: Impact on the onset and progression of metabolic disorders. Int J Mol Sci 20(16)
Petras M, Tatarkova Z, Kovalska M, Mokra D, Dobrota D, Lehotsky J, Drgova A (2014) Hyperhomocysteinemia as a risk factor for the neuronal system disorders. J Physiol Pharmacol 65:15–23
Orellana G, Alvarado L, Muñoz-Neira C, Ávila R, Méndez MF, Slachevsky A (2013) Psychosis-related matricide associated with a lesion of the ventromedial prefrontal cortex. J Am Acad Psychiatry Law 41(3):401–406
Orellana G, Slachevsky A (2013) Executive functioning in schizophrenia. Front Psychiatry 4:1–15
Al-Hakeim HK, Almulla AF, Al-Dujaili AH, Maes M (2020) Construction of a neuro-immune-cognitive pathway-phenotype underpinning the phenome of deficit schizophrenia. Curr Top Med Chem 20(9):747–758
Stoyanov D (2020) The reification of diagnosis in psychiatry. Neurotox Res 37(3):772–774. https://doi.org/10.1007/s12640-019-00139-2
RDoC initiative (2020) Research domain criteria (RDoC), https://www.nimh.nih.gov/research/research-funded-by-nimh/rdoc/index.shtml As assessed 19-7-2020
Stoyanov D, Kandilarova S, Paunova R, Barranco Garcia J, Latypova A, Kherif F (2019) Cross-validation of functional MRI and paranoid-depressive scale: results from multivariate analysis. Front Psychiatry 25:10:869
Acknowledgements
We are thankful for the contribution of the Postgraduate Laboratory of the University Hospital of Londrina and research support given by Asahi Glass Foundation, Chulalongkorn University Centenary Academic Development Project and Ratchadapiseksompotch Funds, Faculty of Medicine, Chulalongkorn University.
Authorships
BK and MM made the design of the study. BK recruited and screened the participants. MM performed statistical analyses. AKM, APM, LOS, JVLP, EGM, SS, and DSB performed the assays. AFC and MS contributed in a meaningful way to the intellectual content of this paper. All authors agreed upon the final version of the paper.
Funding
The study was supported by the Asahi Glass Foundation, Chulalongkorn University Centenary Academic Development Project and Ratchadapiseksompotch Funds, Faculty of Medicine, Chulalongkorn University, grant numbers RA60/042 (to BK) and RA61/050 (to MM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Statement
The study was approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (No 298/57), which is in compliance with the International Guideline for Human Research protection as required by the Declaration of Helsinki, The Belmont Report, CIOMS Guideline, and International Conference on Harmonization on Good Clinical Practice (ICH-GCP).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maes, M., Sirivichayakul, S., Matsumoto, A.K. et al. Lowered Antioxidant Defenses and Increased Oxidative Toxicity Are Hallmarks of Deficit Schizophrenia: a Nomothetic Network Psychiatry Approach. Mol Neurobiol 57, 4578–4597 (2020). https://doi.org/10.1007/s12035-020-02047-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02047-5