Abstract
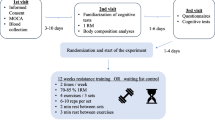
Alzheimer’s disease (AD) is characterized by progressive impairment of memory, with an etiology involving oxidative stress and inflammation. Exercise training is a safe, efficacious, and economic approach to manage neurodegenerative diseases. In AD, the biomarkers of oxidative damage to lipids, proteins, and DNA are elevated. In the present study, we aimed to evaluate whether exercise is effective in patients with AD by assessing the serum biomarkers associated with the redox status, neurotrophin levels, and inflammatory system. This nonrandomized clinical study (n = 15) involved 22 training sessions performed twice a week (60 min/session) in patients diagnosed with AD. The cognitive and self-awareness tests were performed 48 h before and after the physical training session. In patients with AD, physical training significantly improved the judgment and problem-solving domains of the memory score; however, general mental health, memory, orientation, and home/hobby domains were improved slightly, and the neurotrophin levels remained unaltered. Significantly, the markers of protein integrity also increased following exercise. Furthermore, catalase activity and ROS levels decreased, nitrite levels increased, and interleukin-4 level increased following physical training in patients with AD. Although proinflammatory cytokines remained unaltered, the levels of neuron-specific enolase, a marker of neuronal damage, decreased following exercise training in these patients. In conclusion, physical exercise training could be a safe and effective method for blocking the AD progression and improving the antioxidant capacity and anti-inflammatory system, whereas certain assessed biomarkers could be utilized to monitor AD therapy.




Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Dement A (2016) Alzheimer’s disease facts and figures. Alzheimer’s Dement J Alzheimer’s Assoc 12(4):459–509. https://doi.org/10.1016/j.jalz.2016.03.001
Querfurth H, LaFerla F (2010) Alzheimer’s disease. N Engl J Med 329:10
Fratiglioni L, Qiu C (2009) Prevention of common neurodegenerative disorders in the elderly. Exp Gerontol 44(1–2):46–50
Padurariu M, Ciobica A, Hritcu L, Stoica B, Bild W, Stefanescu C (2010) Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer’s disease. Neurosci Lett 469(1):6–10. https://doi.org/10.1016/j.neulet.2009.11.033
Keller J, Schmitt F, Scheff S, Ding Q, Chen Q, Butterfield D et al (2005) Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology 64(7):1152–1156. https://doi.org/10.1212/01.WNL.0000156156.13641.BA
Schrag M, Mueller C, Zabel M, Crofton A, Kirsch W, Ghribi O et al (2013) Oxidative stress in blood in Alzheimer’s disease and mild cognitive impairment: a meta-analysis. Neurobiol Dis 59:100–110. https://doi.org/10.1016/j.nbd.2013.07.005
van der Kleij L, Petersen E, Siebner H, Hendrikse J, Frederiksen K, Sobol N et al (2016) The effect of physical exercise on cerebral blood flow in Alzheimer’s disease: P32004. Eur J Neurol 23:749–750. https://doi.org/10.1016/j.nicl.2018.09.003
Zhu X, Lee H, Casadesus G, Avila J, Drew K, Perry G et al (2005) Oxidative imbalance in Alzheimer’s disease. Mol Neurobiol 31(1–3):205–217. https://doi.org/10.1385/MN:31:1-3:205
Cadenas E, Davies KJ (2000) Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29(3–4):222–230. https://doi.org/10.1016/S0891-5849(00)00317-8
Hajjar I, Hayek SS, Goldstein FC, Martin G, Jones DP, Quyyumi A (2018) Oxidative stress predicts cognitive decline with aging in healthy adults: an observational study. J Neuroinflammation 15(1):1–7. https://doi.org/10.1186/s12974-017-1026-z
Verdile G, Keane KN, Cruzat VF, Medic S, Sabale M, Rowles J, et al (2015) Inflammation and oxidative stress: the molecular connectivity between insulin resistance, obesity, and Alzheimer’s disease. Mediators Inflamm 2015. https://doi.org/10.1155/2015/105828
Kamat PK, Kalani A, Rai S, Swarnkar S, Tota S, Nath C et al (2016) Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of Alzheimer’s disease: understanding the therapeutics strategies. Mol Neurobiol 53(1):648–661. https://doi.org/10.1007/s12035-014-9053-6
Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G (2000) Inflamm- Aging Evol Perspect Immunosenescence. Ann NY Acad Sci 908:244–254. https://doi.org/10.1111/j.1749-6632.2000.tb06651.x
Chen W, Zhang X, Huang W (2016) Role of physical exercise in Alzheimer’s disease. Biomed Rep 4(4):403–407. https://doi.org/10.3892/br.2016.607
Kennedy G, Hardman RJ, Macpherson H, Scholey AB, Pipingas A (2017) How does exercise reduce the rate of age-associated cognitive decline? A review of potential mechanisms. J Alzheimers Dis 55(1):1–18. https://doi.org/10.3233/JAD-160665
Chen Z, Qin X, Zhang X, Liu B, Chen M (2020) Upregulation of IL-4 signaling contributes to aerobic exercise-induced insulin sensitivity. Biochem Biophys Res Commun. https://doi.org/10.1016/j.bbrc.2020.02.103
Ryan SM, Nolan YM (2016) Neuroinflammation negatively affects adult hippocampal neurogenesis and cognition: can exercise compensate? Neurosci Biobehav Rev 61:121–131. https://doi.org/10.1016/j.neubiorev.2015.12.004
Kramer AF, Erickson KI, Colcombe SJ (2006) Exercise, cognition, and the aging brain. J Appl Physiol 101(4):1237–1242. https://doi.org/10.1152/japplphysiol.00500.2006
Teri L, Logsdon RG, McCurry SM (2008) Exercise interventions for dementia and cognitive impairment: the Seattle Protocols. J Nutr Health Aging 12(6):391–394. https://doi.org/10.1007/BF02982672
Christofoletti G, Oliani MM, Gobbi S, Stella F, Bucken Gobbi LT, Renato CP (2008) A controlled clinical trial on the effects of motor intervention on balance and cognition in institutionalized elderly patients with dementia. Clin Rehabil 22(7):618–626. https://doi.org/10.1177/0269215507086239
Hoffmann K, Sobol NA, Frederiksen KS, Beyer N, Vogel A, Vestergaard K et al (2016) Moderate-to-high intensity physical exercise in patients with Alzheimer’s disease: a randomized controlled trial. J Alzheimers Dis 50(2):443–453. https://doi.org/10.3233/JAD-150817
Cotman CW, Berchtold NC, Christie L-A (2007) Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci 30(9):464–472. https://doi.org/10.1016/j.tins.2007.06.011
Muller AP, Gnoatto J, Moreira JD, Zimmer ER, Haas CB, Lulhier F et al (2011) Exercise increases insulin signaling in the hippocampus: physiological effects and pharmacological impact of intracerebroventricular insulin administration in mice. Hippocampus 21(10):1082–1092. https://doi.org/10.1002/hipo.20822
Muller AP, Zimmer ER, Kalinine E, Haas CB, Oses JP, Martimbianco de Assis A et al (2012) Physical exercise exacerbates memory deficits induced by intracerebroventricular STZ but improves insulin regulation of H 2O 2 production in mice synaptosomes. J Alzheimers Dis 30(4):889–898. https://doi.org/10.3233/JAD-2012-112066
Medhat E, Rashed L, Abdelgwad M, Aboulhoda BE, Khalifa MM, El-Din SS (2020) Exercise enhances the effectiveness of vitamin D therapy in rats with Alzheimer’s disease: emphasis on oxidative stress and inflammation. Metab Brain Dis 35(1):111–120. https://doi.org/10.1007/s11011-019-00504-2
Özbeyli D, Sarı G, Özkan N, Karademir B, Yüksel M, Kaya ÖTÇ et al (2017) Protective effects of different exercise modalities in an Alzheimer’s disease-like model. Behav Brain Res 328:159–177. https://doi.org/10.1016/j.bbr.2017.03.044
De la Rosa A, Solana E, Corpas R, Bartrés-Faz D, Pallàs M, Vina J et al (2019) Long-term exercise training improves memory in middle-aged men and modulates peripheral levels of BDNF and Cathepsin B. Sci Rep 9(1):1–11. https://doi.org/10.1038/s41598-019-40040-8
Bernardo T, Marques-Aleixo I, Beleza J, Oliveira P, Ascensao A, Magalhaes J (2016) Physical exercise and brain mitochondrial fitness: the possible role against Alzheimer’s disease. Brain Pathol 26(5):648–663. https://doi.org/10.1111/bpa.12403
Islam MT (2017) Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol Res 39(1):73–82. https://doi.org/10.1080/01616412.2016.1251711
Isgrò M, Bottoni P, Scatena R (2015) Advances in cancer biomarkers: from biochemistry to clinic for a critical revision. https://doi.org/10.1007/978-94-017-7215-0
Cunha JA (2001) Manual da versão em português das Escalas Beck. São Paulo Casa Psicólogo 256
Brucki S, Nitrini R, Caramelli P, Bertolucci PH, Okamoto IH (2003) Sugestões para o uso do mini-exame do estado mental no Brasil. Arq Neuropsiquiatr 61(3B):777–781. https://doi.org/10.1590/S0004-282X2003000500014
Hughes CP, Berg L, Danziger W, Coben LA, Martin RL (1982) A new clinical scale for the staging of dementia. Br J Psychiatry 140(6):566–572. https://doi.org/10.1192/bjp.140.6.566
Morris J (1993) Current vision and scoring rules the clinical dementia rating (CDR). Neurology 43:2412–2414. https://doi.org/10.1212/WNL.43.11.2412-a
Ware Jr JE, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care 473–83
Hempel SL, Buettner GR, O’Malley YQ, Wessels DA, Flaherty DM (1999) Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: comparison with 2′, 7′-dichlorodihydrofluorescein diacetate, 5 (and 6)-carboxy-2′, 7′-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic Biol Med 27(1–2):146–159. https://doi.org/10.1016/S0891-5849(99)00061-1
Chae SY, Lee M, Kim SW, Bae YH (2004) Protection of insulin secreting cells from nitric oxide induced cellular damage by crosslinked hemoglobin. Biomaterials 25(5):843–850. https://doi.org/10.1016/S0142-9612(03)00605-7
Aebi H (1984) [13] Catalase in vitro. In: Methods in enzymology. Elsevier. p. 121–6. https://doi.org/10.1016/S0076-6879(84)05016-3
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz A-G et al (1990) [49] Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478. https://doi.org/10.1016/0076-6879(90)86141-H
Bhatti GK, Reddy AP, Reddy PH, Bhatti JS (2020) Lifestyle modifications and nutritional interventions in aging-associated cognitive decline and Alzheimer’s disease. Front Aging Neurosci 11:369. https://doi.org/10.3389/fnagi.2019.00369
Zanco M, Placido J, Marinho V, Ferreira JV, de Oliveira F, Monteiro-Junior R et al (2018) Spatial navigation in the elderly with Alzheimer’s disease: a cross-sectional study. J Alzheimers Dis 66(4):1683–1694. https://doi.org/10.3233/JAD-180819
Binder EF, Storandt M, Birge SJ (1999) The relation between psychometric test performance and physical performance in older adults. J Gerontol Ser Biomed Sci Med Sci 54(8):M428–M432. https://doi.org/10.1093/gerona/54.8.M428
Weller J, Budson A (2018) Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 7. https://doi.org/10.12688/f1000research.14506.1
Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM et al (2007) An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci 104(13):5638–5643. https://doi.org/10.1073/pnas.0611721104
Strüder H, Weicker H (2001) Physiology and pathophysiology of the serotonergic system and its implications on mental and physical performance. Part I. Int J Sports Med 22(07):467–481. https://doi.org/10.1055/s-2001-17605
Vilela TC, Muller AP, Damiani AP, Macan TP, da Silva S, Canteiro PB et al (2017) Strength and aerobic exercises improve spatial memory in aging rats through stimulating distinct neuroplasticity mechanisms. Mol Neurobiol 54(10):7928–7937. https://doi.org/10.1007/s12035-016-0272-x
Bortz W 2nd (1981) Catecholamines, dopamine, and endorphin levels during extreme exercise. N Engl J Med 305:466–467
Belviranlı M, Okudan N (2019) Voluntary, involuntary and forced exercises almost equally reverse behavioral impairment by regulating hippocampal neurotrophic factors and oxidative stress in experimental Alzheimer’s disease model. Behav Brain Res 364:245–255. https://doi.org/10.1016/j.bbr.2019.02.030
Bhattarai P, Cosacak MI, Mashkaryan V, Demir S, Popova SD, Govindarajan N et al (2020) Neuron-glia interaction through Serotonin-BDNF-NGFR axis enables regenerative neurogenesis in Alzheimer’s model of adult zebrafish brain. PLoS Biol 18(1):e3000585. https://doi.org/10.1371/journal.pbio.3000585
Khan MZ, Zhuang X, He L (2016) GPR40 receptor activation leads to CREB phosphorylation and improves cognitive performance in an Alzheimer’s disease mouse model. Neurobiol Learn Mem 131:46–55. https://doi.org/10.1016/j.nlm.2016.03.006
Nunomura A, Tamaoki T, Motohashi N, Nakamura M, McKeel DW Jr, Tabaton M et al (2012) The earliest stage of cognitive impairment in transition from normal aging to Alzheimer disease is marked by prominent RNA oxidation in vulnerable neurons. J Neuropathol Exp Neurol 71(3):233–241. https://doi.org/10.1097/NEN.0b013e318248e614
Butterfield DA, Halliwell B (2019) Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci 20(3):148–160. https://doi.org/10.1038/s41583/-019-0132-6
Torres LL, Quaglio NB, de Souza GT, Garcia RT, Dati LMM, Moreira WL et al (2011) Peripheral oxidative stress biomarkers in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis 26(1):59–68. https://doi.org/10.3233/JAD-2011-110284
Kaufmann FN, Gazal M, Mondin TC, Cardoso TA, Quevedo LÁ, Souza LD et al (2015) Cognitive psychotherapy treatment decreases peripheral oxidative stress parameters associated with major depression disorder. Biol Psychol 110:175–181. https://doi.org/10.1016/j.biopsycho.2015.08.001
Bermejo P, Martín-Aragón S, Benedí J, Susín C, Felici E, Gil P et al (2008) Peripheral levels of glutathione and protein oxidation as markers in the development of Alzheimer’s disease from mild cognitive impairment. Free Radic Res 42(2):162–170. https://doi.org/10.1080/10715760701861373
Hensley K, Hall N, Subramaniam R, Cole P, Harris M, Aksenov M et al (1995) Brain regional correspondence between Alzheimer’s disease histopathology and biomarkers of protein oxidation. J Neurochem 65(5):2146–2156. https://doi.org/10.1046/j.1471-4159.1995.65052146.x
Smith C, Carney JM, Starke-Reed P, Oliver C, Stadtman E, Floyd R et al (1991) Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci 88(23):10540–10543. https://doi.org/10.1073/pnas.88.23.10540
Ahmad W, Ijaz B, Shabbiri K, Ahmed F, Rehman S (2017) Oxidative toxicity in diabetes and Alzheimer’s disease: mechanisms behind ROS/RNS generation. J Biomed Sci 24(1):76. https://doi.org/10.1186/s12929-017-0379-z
Newsholme P (2010) Homem De Bittencourt Jr PI, O’Hagan C, De Vito G, Murphy C, Krause MS (2010) Exercise and possible molecular mechanisms of protection from vascular disease and diabetes: the central role of ROS and nitric oxide. Clin Sci 118(5):341–349. https://doi.org/10.1042/CS20090433
Mangialasche F, Polidori MC, Monastero R, Ercolani S, Camarda C, Cecchetti R et al (2009) Biomarkers of oxidative and nitrosative damage in Alzheimer’s disease and mild cognitive impairment. Ageing Res Rev 8(4):285–305. https://doi.org/10.1016/j.arr.2009.04.002
Sharma S, Verma S, Kapoor M, Saini A, Nehru B (2016) Alzheimer’s disease like pathology induced six weeks after aggregated amyloid-beta injection in rats: increased oxidative stress and impaired long-term memory with anxiety-like behavior. Neurol Res 38(9):838–850. https://doi.org/10.1080/01616412.2016.1209337
Lundberg JO, Weitzberg E, Gladwin MT (2008) The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7(2):156–167. https://doi.org/10.1038/nrd2466
Calvert JW (2011) Cardioprotective effects of nitrite during exercise. Cardiovasc Res 89(3):499–506. https://doi.org/10.1093/cvr/cvq307
Ferreira ST, Clarke JR, Bomfim TR, De Felice FG (2014) Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer’s disease. Alzheimers Dement 10:S76-83. https://doi.org/10.1016/j.jalz.2013.12.010
Jiang NM, Cowan M, Moonah SN, Petri WA Jr (2018) The impact of systemic inflammation on neurodevelopment. Trends Mol Med 24(9):794–804. https://doi.org/10.1016/j.molmed.2018.06.008
Ismail R, Parbo P, Madsen LS, Hansen AK, Hansen KV, Schaldemose JL et al (2020) The relationships between neuroinflammation, beta-amyloid and tau deposition in Alzheimer’s disease: a longitudinal PET study. J Neuroinflammation 17:1–11. https://doi.org/10.1186/s12974-020-01820-6
Casella G, Garzetti L, Gatta AT, Finardi A, Maiorino C, Ruffini F et al (2016) IL4 induces IL6-producing M2 macrophages associated to inhibition of neuroinflammation in vitro and in vivo. J Neuroinflammation 13(1):1–10. https://doi.org/10.1186/s12974-016-0596-5
da Luz Scheffer D, Latini A (2020) Exercise-induced immune system response: anti-inflammatory status on peripheral and central organs. Biochim Biophys Acta BBA-Mol Basis Dis 165823. https://doi.org/10.1016/j.bbadis.2020.165823
Pahk K, Kim EJ, Joung C, Seo HS, Kim S (2020) Exercise training reduces inflammatory metabolic activity of visceral fat assessed by 18F‐FDG PET/CT in obese women. Clin Endocrinol (Oxf). https://doi.org/10.1111/cen.14216
Italiani P, Puxeddu I, Napoletano S, Scala E, Melillo D, Manocchio S et al (2018) Circulating levels of IL-1 family cytokines and receptors in Alzheimer’s disease: new markers of disease progression? J Neuroinflammation 15(1):1–12. https://doi.org/10.1186/s12974-018-1376-1
Schmidt FM, Mergl R, Stach B, Jahn I, Gertz H-J, Schönknecht P (2014) Elevated levels of cerebrospinal fluid neuron-specific enolase (NSE) in Alzheimer’s disease. Neurosci Lett 570:81–85. https://doi.org/10.1016/j.neulet.2014.04.007
Hoffmann J, Janowitz D, Van der Auwera S, Wittfeld K, Nauck M, Friedrich N et al (2017) Association between serum neuron-specific enolase, age, overweight, and structural MRI patterns in 901 subjects. Transl Psychiatry 7(12):1–9. https://doi.org/10.1038/s41398-017-0035-0
Chaves ML, Camozzato AL, Ferreira ED, Piazenski I, Kochhann R, Dall’Igna O, et al (2010) Serum levels of S100B and NSE proteins in Alzheimer’s disease patients. J Neuroinflammation 7(1):6. https://doi.org/10.1186/1742-2094-7-6
Olsson B, Lautner R, Andreasson U, Öhrfelt A, Portelius E, Bjerke M et al (2016) CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet NeuroL 1 de junho de 15(7):673–684. https://doi.org/10.1016/S1474-4422(16)00070-3
Funding
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)-Instituto Nacional de Neurociência Translacional-INNT #465346/2014–6, Projeto CNPq UNIVERSAL 2018, Fundação de Amparo a Pesquisa do Estado de Santa Catarina (FAPESC)-PPSUS-2016 e Universidade do Extremo Sul Catarinense (UNESC).
Author information
Authors and Affiliations
Contributions
Farias JM: conceptualization, data curation, investigation, methodology, project administration, supervision, writing original manuscript draft. Tramontin NS: investigation, methodology, blood collection, biochemical analyses. Pereira EV: investigation, methodology, physical training, behavioral tasks. Moraes GL: investigation, methodology, physical training, behavioral tasks. Furtado BG: investigation, methodology, physical training, behavioral tasks. Tietbohl LTW: investigation, methodology, blood collection, biochemical analyses. Pereira BC: investigation, methodology, blood collection, biochemical analyses. Simon KU: investigation, methodology, blood collection, biochemical analyses. Muller AP: conceptualization, data curation, investigation, methodology, project administration, supervision, writing original manuscript draft.
Corresponding author
Ethics declarations
Ethics Approval
Resolution 466/12 of the National Health Council, under number 3.034.010.
Consent to Participate
See attached.
Consent for Publication
All authors have read the final version of the manuscript and given their consent for publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 64.3 kb)
Rights and permissions
About this article
Cite this article
de Farias, J.M., dos Santos Tramontin, N., Pereira, E.V. et al. Physical Exercise Training Improves Judgment and Problem-Solving and Modulates Serum Biomarkers in Patients with Alzheimer’s Disease. Mol Neurobiol 58, 4217–4225 (2021). https://doi.org/10.1007/s12035-021-02411-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02411-z