Abstract
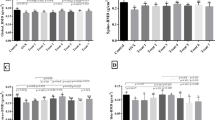
Osteoporosis is a major health problem that occurs as a result of an imbalance between bone formation and bone resorption. Different approaches have been established for treating osteoporosis. Recently, because of their health benefits and also low adverse reaction, probiotics have been receiving considerable attention. In this study, we compared the effectiveness of five probiotic strains, Lactobacillus acidophilus, Lactobacillus reuteri, Lactobacillus casei, Bifidobacterium longum, and Bacillus coagulans, in protecting rats from ovariectomized (OVX)-induced bone loss. Forty-nine adult female Sprague-Dawley rats were allocated into seven groups as follows: group 1, control; group 2, OVX; group 3, OVX + Lactobacillus acidophilus; group 4, OVX + Lactobacillus casei; group 5, OVX + Bacillus coagulans; group 6, OVX + Bifidobacterium longum; and group 7, OVX + Lactobacillus reuteri. Probiotics were fed to OVX groups at the concentration of (1 × 109 CFU/ml/day) for 4 weeks. Then, biochemical parameters, including vitamin D, calcium (Ca), phosphorus (P), and alkaline phosphatase (ALP), were assessed. Dual-energy X-ray absorptiometry (DEXA) scans were used that assess bone mineral density (BMD), bone marrow concentration (BMC), and area of global, femur, spine, and tibia. Lactobacillus acidophilus and Lactobacillus casei significantly increased Ca and ALP and decreased P in treated groups. Lactobacillus casei, Lactobacillus reuteri, and Bifidobacterium longum increased vitamin D significantly. Lactobacillus acidophilus and Lactobacillus casei indicated the most effects on BMD. In terms of BMC, and bone area, Lactobacillus acidophilus, Lactobacillus reuteri, and Lactobacillus casei demonstrated the significant enhancement in OVX groups treated with. Among the probiotics used in this study, Lactobacillus acidophilus and Lactobacillus casei showed the most effects in terms of BMD, BMC, bone area, and biochemical parameters. It seems that probiotics effects on bone health are strain dependent, but further studies should be done to prove these findings.




Similar content being viewed by others
References
Vandenbroucke A et al (2017) Pharmacological treatment of osteoporosis in the oldest old. Clin Interv Aging 12:1065–1077
McCabe LR, Irwin R, Schaefer L, Britton RA (2013) Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J Cell Physiol 228:1793–1798
Compston J et al (2017) UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos 12:43
Parvaneh K et al (2015) Probiotics (Bifidobacterium longum) increase bone mass density and upregulate Sparc and Bmp-2 genes in rats with bone loss resulting from ovariectomy. Biomed Res Int 2015:897639
Li JY, Chassaing B, Tyagi AM, Vaccaro C, Luo T, Adams J, Darby TM, Weitzmann MN, Mulle JG, Gewirtz AT, Jones RM, Pacifici R (2016) Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest 126:2049–2063
Britton RA, Irwin R, Quach D, Schaefer L, Zhang J, Lee T, Parameswaran N, McCabe LR (2014) Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol 229:1822–1830
Weaver CM (2015) Diet, gut microbiome, and bone health. Curr Osteoporos Rep 13:125–130
Shokri D, Khorasgani MR, Mohkam M, Fatemi SM, Ghasemi Y, Taheri-Kafrani A (2018) The inhibition effect of lactobacilli against growth and biofilm formation of Pseudomonas aeruginosa. Probiotics Antimicrob Proteins 10:34–42
Mohkam M et al (2016) Characterization and in vitro probiotic assessment of potential indigenous Bacillus strains isolated from soil rhizosphere. Minerva Biotec 28:19–28
Hemarajata P, Versalovic J (2013) Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Ther Adv Gastroenterol 6:39–51
Grazul H, Kanda LL, Gondek D (2016) Impact of probiotic supplements on microbiome diversity following antibiotic treatment of mice. Gut Microbes 7:101–114
Scholz-Ahrens KE, Ade P, Marten B, Weber P, Timm W, Aςil Y, Glüer CC, Schrezenmeir J (2007) Prebiotics, probiotics, and synbiotics affect mineral absorption, bone mineral content, and bone structure. J Nutr 137:838s–846s
Legette LL, Lee WH, Martin BR, Story JA, Arabshahi A, Barnes S, Weaver CM (2011) Genistein, a phytoestrogen, improves total cholesterol, and synergy, a prebiotic, improves calcium utilization, but there were no synergistic effects. Menopause 18:923–931
Parvaneh K et al (2014) Effect of probiotics supplementation on bone mineral content and bone mass density. Sci World J 2014:5959–5962
Chiang SS, Pan TM (2011) Antiosteoporotic effects of Lactobacillus-fermented soy skim milk on bone mineral density and the microstructure of femoral bone in ovariectomized mice. J Agric Food Chem 59:7734–7742
Li F, Jiang X, Zhao J, Zhang S (2015) Graphene oxide: a promising nanomaterial for energy and environmental applications. Nano Energy 16:488–515
Narva M, Collin M, Lamberg-Allardt C, Kärkkäinen M, Poussa T, Vapaatalo H, Korpela R (2004) Effects of long-term intervention with Lactobacillus helveticus-fermented milk on bone mineral density and bone mineral content in growing rats. Ann Nutr Metab 48:228–234
Scholz-Ahrens KE, Adolphi B, Rochat F, Barclay DV, de Vrese M, Açil Y, Schrezenmeir J (2016) Effects of probiotics, prebiotics, and synbiotics on mineral metabolism in ovariectomized rats—impact of bacterial mass, intestinal absorptive area and reduction of bone turn-over. NFS J 3:41–50
Chang Y, Yang ST, Liu JH, Dong E, Wang Y, Cao A, Liu Y, Wang H (2011) In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol Lett 200:201–210
Thomas DW, Greer FR (2010) Probiotics and prebiotics in pediatrics. Pediatrics 126:1217–1231
Kimoto-Nira H, Suzuki C, Kobayashi M, Sasaki K, Kurisaki J, Mizumachi K (2007) Anti-ageing effect of a lactococcal strain: analysis using senescence-accelerated mice. Br J Nutr 98:1178–1186
Mutus R et al (2006) The effect of dietary probiotic supplementation on tibial bone characteristics and strength in broilers. Poult Sci 85:1621–1625
McCabe L, Britton RA, Parameswaran N (2015) Prebiotic and probiotic regulation of bone health: role of the intestine and its microbiome. Curr Osteoporos Rep 13:363–371
Sjogren K et al (2012) The gut microbiota regulates bone mass in mice. J Bone Miner Res 27:1357–1367
Kumar ASK, Jiang S-J (2016) Chitosan-functionalized graphene oxide: a novel adsorbent an efficient adsorption of arsenic from aqueous solution. J Environ Chem Eng 4:1698–1713
Amdekar S, Kumar A, Sharma P, Singh R, Singh V (2012) Lactobacillus protected bone damage and maintained the antioxidant status of liver and kidney homogenates in female wistar rats. Mol Cell Biochem 368:155–165
Ohlsson C, Engdahl C, Fåk F, Andersson A, Windahl SH, Farman HH, Movérare-Skrtic S, Islander U, Sjögren K (2014) Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One 9:e92368
Rodrigues FC, Castro ASB, Rodrigues VC, Fernandes SA, Fontes EAF, de Oliveira TT, Martino HSD, de Luces Fortes Ferreira CL (2012) Yacon flour and Bifidobacterium longum modulate bone health in rats. J Med Food 15:664–670
Campbell JM, Fahey GC Jr, Wolf BW (1997) Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. J Nutr 127:130–136
Villa ML, Marcus R, Ramírez Delay R, Kelsey JL (1995) Factors contributing to skeletal health of postmenopausal Mexican-American women. J Bone Miner Res 10:1233–1242
Gilman J, Cashman KD (2006) The effect of probiotic bacteria on transepithelial calcium transport and calcium uptake in human intestinal-like Caco-2 cells. Curr Issues Intest Microbiol 7:1–5
Pérez-Conesa D, López G, Abellán P, Ros G (2006) Bioavailability of calcium, magnesium and phosphorus in rats fed probiotic, prebiotic and synbiotic powder follow-up infant formulas and their effect on physiological and nutritional parameters. J Sci Food Agric 86:2327–2336
Mora-Gutierrez A, Farrell HM Jr, Attaie R, McWhinney VJ, Wang C (2007) Effects of bovine and caprine Monterey Jack cheeses fortified with milk calcium on bone mineralization in rats. Int Dairy J 17:255–267
Law YY, Chiu HF, Lee HH, Shen YC, Venkatakrishnan K, Wang CK (2016) Consumption of onion juice modulates oxidative stress and attenuates the risk of bone disorders in middle-aged and post-menopausal healthy subjects. Food Funct 7:902–912
Xu X et al (2017) Intestinal microbiota: a potential target for the treatment of postmenopausal osteoporosis. Bone Res 5:201746–201764
Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, Sartor BR, Aliprantis AO, Charles JF (2016) Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci 113:E7554–E7563
Xu X, Jia X, Mo L, Liu C, Zheng L, Yuan Q, Zhou X (2017) Intestinal microbiota: a potential target for the treatment of postmenopausal osteoporosis. Bone Res 5:17046
Dabbaghmanesh MH, Noorafshan A, Talezadeh P, Tanideh N, Koohpeyma F, Iraji A, Bakhshayeshkaram M, Montazeri-Najafabady N (2017) Stereological investigation of the effect of Elaeagnus angustifolia fruit hydroalcoholic extract on osteoporosis in ovariectomized rats. Avicenna J Phytomed 7:261–274
Cao H, Zhang Y, Qian W, Guo XP, Sun C, Zhang L, Cheng XH (2017) Effect of icariin on fracture healing in an ovariectomized rat model of osteoporosis. Exp Ther Med 13:2399–2404
Kim JG, Lee E, Kim SH, Whang KY, Oh S, Imm JY (2009) Effects of a Lactobacillus casei 393 fermented milk product on bone metabolism in ovariectomised rats. Int Dairy J 19:690–695
Acknowledgments
The authors would like to thank the Vice Chancellery of Shiraz University of Medical Sciences. Also, we would like to thank Dr. Milad Mohkam and Dr. Reza Heydari for their valuable comments to improve the quality of the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This work was approved by the Ethics Committee (No. 2225b125) of Shiraz University of Medical Sciences, Shiraz, Iran.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Montazeri-Najafabady, N., Ghasemi, Y., Dabbaghmanesh, M.H. et al. Supportive Role of Probiotic Strains in Protecting Rats from Ovariectomy-Induced Cortical Bone Loss. Probiotics & Antimicro. Prot. 11, 1145–1154 (2019). https://doi.org/10.1007/s12602-018-9443-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-018-9443-6