Abstract
Nanobiotechnology, the bio-branch of nanotechnology is considered to be one of the fastest emerging research fields. Biosynthesis of metallic nanoparticles is currently under exploitation. Use of plant and plant materials for the synthesis of Zinc nanoparticles is relatively new and exciting research field. The biogenic zinc nanoparticles were synthesized using the leaves of Parthenium hysterophorous by green synthesis route. UV–VIS absorption spectroscopy was used to monitor the quantitative formation of zinc nanoparticles. The characteristics of the synthesized zinc nanoparticles were studied using scanning electron microscopy and nanoparticle analyzer. Zinc nanoparticles were observed to be spherical in shape with size range of 16 to 108.5 nm. The measured zeta potentials varied from 100.4 to 117.20 mV indicate high dispersion of the zinc nanoparticles. The synthesized zinc nanoparticles showed good enzymatic activity and microbial activity. The physiological parameters increased from 30 to 60 days of sowing when compared to control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanoscience is about creating new chemical and biological nanostructures, uncovering and understanding their novel properties, and ultimately about learning how to organize these new structures into larger and more complex functional structures and devices. The applications of nanomaterials in agriculture and food sector make all the more relevance and importance since the Indian economy is predominantly depending on agriculture to feed the large population. The nano-sized particles increase the potency of active ingredients and potentially reduce the quantity to be applied. Nano-structured particles/matrix make soil binder similar to natural crop and plant residues providing dust control, soil moisture retention and re-vegetation stimulant for seeding efforts, which simultaneously increase the production while lowering product cost. Indeed, over the past several years, plants, algae, fungi, bacteria, and viruses have been used for production of low-cost, energy-efficient, and non-toxic metallic nanoparticles (Parikh 2010).
Green Synthesis of metallic nanoparticles is eco-friendly. Biosynthetic methods employing either biological microorganisms or plant extracts have emerged as a simple alternative to physical and chemical methods. Plant and plant materials have become a potential source for the synthesis of metallic nanoparticles recently. A number of researchers have reported on synthesis of silver and gold nanoparticles using different plant materials (Sharma et al. 2007, Badri Narayanan and Sakthivel 2008; Badri Narayanan and Sakthive 2010; Philip 2010; 2011). Synthesis of Zinc oxide nanoparticles is also in progress (Kumar et al. 2011; Singh et al. 2011; Gunalan et al. 2012). But till now there is no report on the synthesis of zinc nanoparticles using plant materials. To the best of our knowledge this is the first report on the green synthesis of zinc nanoparticles. The reason for selecting zinc is—zinc, an essential micronutrient plays an important role in the physiological functions of plants. Plants can easily uptake zinc (rhizosphere). Utilization of zinc in tissues is called internal efficiency and uptake of zinc is called external efficiency. Particle size may affect agronomic effectiveness of zinc fertilizers (Prasad et al. 2012).
Parthenium hysterophorous is a species of flowering plant in the aster family, Asteraceae. In India, it is locally known as Congress weed. Coming to the toxicity of the leaves, contact with this plant causes dermatitis and respiratory malfunction in humans, dermatitis in cattle and domestic animals, due to the presence of toxin parthenin. Parthenin plays a pivotal role as root exudate in allelopathic interference with surrounding plants (Belz et al. 2007). Although Parthenium is considered as toxic plant, it is useful for many medicinal, allelopathic and industrial applications (Oudhia 2001).
The main objective of the proposed work is to synthesize the zinc nanoparticles in biological route and the application of these biogenic zinc nanoparticles on agricultural crops by conducting the pot culture experiments to observe the variations in enzyme activity, microbial activity and physiological traits of the plants by taking the soil from the pot cultures and to make a comparative study on the application of zinc nanoparticles applied plants and the plants grown under normal crop conditions (by maintaining controls) to test the potential of green synthesized zinc nanoparticles.
Materials and method
Collection of plant materials
Fresh leaves of Parthenium hysterophorous were collected from the fields of Regional Agricultural Research Station, Acharya Nagarjuna Ranga Agricultural University, Tirupati, India. The leaves were thoroughly washed for 2–3 times with distilled water and then kept shade dried for 5 days to free from moisture. After shade drying, the leaves were kept in hot air oven at 50° C for 5–10 min. Then the leaves were subjected to grinding.
Preparation of leaf extract and samples
The ground powder was again sieved (0.5 mm) for three times. After getting the fine powder plant decoction was made by adding distilled water. The decoction was heated under hot plate for 15 min at 60° C. Then the decoction was filtered using Whatmann No.1 filter paper to get the clear filtered solution of the plant extract. To the plant extract, 1 molar zinc nitrate solution was added in the ratio of 1:19. Two samples were prepared by diluting the first sample (sample-1, 100 %) to its half (sample-2, 50 %). These solutions were kept for heating up to 10–15 min and a change in the color was observed from pale yellow color to dark honey color at 65 °C. After the color change, the samples were purified by centrifugation at 18G for 15 min.
Recording localized surface plasmon resonance (LSPR)
UV–Visible Spectrophotometer (Schimadzou 4650) was used to record LSPR of zinc nanoparticles. Wavelength verses absorption spectra were recorded for the two samples. Surface Plasmon Resonance (SPR) in nanometer-sized structures is called localized surface plasmon resonance. SPR is the basis of many standard tools for measuring adsorption of material onto planar metal surfaces or onto the surface of metal (Zeng et al. 2011). It is the fundamental principle behind many color-based biosensor applications. LSPRs are collective electron charge oscillations in metallic nanoparticles that are excited by light. They exhibit enhanced near-field amplitude at the resonance wavelength.
Particle size and zeta potential measurement
The zeta potentials were recorded using Particle size analyzer (Horiba Nanoparticula 100). This apparatus uses Dynamic Light Scattering (DLS) phenomenon. The sample holder temperature was maintained at 25° C, a technique used to determine the size distribution profile of small particles in solution. This measurement depends on the size of the particle core, the size of surface structures, particle concentration, and the type of ions in the medium.
Fourier transform infrared spectroscopic (FTIR) studies
FTIR spectrum (Bruker Tensor 27) was recorded in mid IR region in the range of 400–4,000 wavenumber (cm−1). A drop of the sample solution (sample-1) was carefully added to the potassium bromide crystals using 1 ml micropipette and the spectrum was recorded in transmittance (%) mode. From the FTIR analysis, the presence of functional groups can be identified. The biological components interact with the metal salts via these functional groups and undergo bio-reduction into nanoparticles (Ganesh Babu and Gunasekaran 2009).
Scanning electron microscope (SEM) studies
The particle size and shape can be identified using the scanning electron microscope (Carlziess EVO 50). For the preparation of sample for SEM, sample-1 was coated on a thick aluminum sheet cut into 1 × 1 cm width. This sheet was kept on hot plate and the temperature was maintained at 65° C. 0.5 ml of sample was taken into the micropipette and carefully poured on to the aluminum sheet. This step was repeated for three times. Like this, a smear was obtained on the sheet. This was taken for the SEM analysis.
Pot culture experiment
Arachis hypogea L, one of the important oil seeds crops of India grown in rainfed soils was selected for pot culture. The soil pH was measured as 6.8. 20 pots (15 × 12 × 12 cm dimension for each pot) were raised as five replications, for which, 15 pots were added with sample-1 in three different variations in three treatments and five pots were maintained as controls without adding any sample. The bacteria, fungi and actinomycetes population were analyzed from soil samples using serial dilution plating technique after 60 days of sowing. The population was expressed as cfu g−1 of oven dry soil. Phosphatase and dehydrogenase enzymatic activities were estimated by adopting standard methods, after 30 and 60 days of sowing.
Physiological parameters like plant height, number of leaves and leaf surface ratio (leaf length and breadth) were calculated in two time intervals of 30 and 60 days of sowing. Similarly, root length, shoot length, fresh and dry weight of roots and shoots and total biomass (dry weight of root and shoot) were estimated after 60 days of sowing. The total biomass was calculated after removing the plant from all the pots and by separating each plant into its root and shoot. This was followed by measuring the root length and shoot length and weighing of fresh and dry weights of all the roots and shoots. The fresh weights were measured immediately after removing the plant from the pots and the dry weights were measured after incubating the roots and shoots in an oven for 36 h at 60 °C.
Results and discussion
Ultraviolet–visible spectroscopy
Zinc nanoparticles were characterized by studying the absorption spectra recorded from the Ultraviolet–visible spectral analysis for the two samples (Table 1). Formation of zinc nanoparticles in aqueous colloidal solution were confirmed using Ultraviolet–visible spectral analysis. Zinc nanoparticles normally show a broad peak in the UV–vis spectrum in the range of 230–330 nm (Revina et al. 2007). The optical transitions have been observed at 327.50 and 330.00 nm (Fig. 1a, b) corresponds to the formation of zinc nanoparticles. The absorbance values at different wavelengths are given in Table 1. Between the two samples, highest absorbance of 0.373 A % at 330.00 nm was observed for sample-1 because of higher concentration compared to sample-2. The bio matrix present in the plant extracts may leads to the change in the absorbance of UV–vis spectra.
The results show promise for the development of a “green” process for nanoparticle synthesis. Biomolecules found in plants induce the reduction of Zn2+ ions into Zn nanoparticles. The process of reduction is extracellular and fast leading to the development of easy biosynthesis of zinc nanoparticles. At the time of breakdown of zinc nitrate into zinc and nitrate ions, a nitrate reductase enzyme makes the reaction to take place.
The zinc content was estimated from Inductively Coupled Plasma-Optical Emission Spectrophotometer (ICP-OES—Leeman Labs Prodigy XP) as 65 ppm (Wolf et al. 2009).
Nanoparticle analyzer and SEM studies
From the nanoparticle analyzer, the zeta potential was measured as 117.20 mV for sample-1 and 100.40 mV for sample-2 (Fig. 2a, b) and particle size was measured as 16.10 nm for sample-1 and 58.60 nm for sample-2 (Fig. 3a, b). The significance of zeta potential is that its value can be related to the stability of colloidal dispersions. The zeta potential indicates the degree of repulsion between adjacent and similarly charged particles in dispersion. Zeta potential indicates the stability of the nanoparticles. Here, the zeta potential value indicated good stability with high potential.
From Table 1, highest zeta potential of 117.20 mV and least particle size of 16.10 nm were observed for sample-1 (higher concentration than sample-2 as sample-2 is the diluted form of sample-1) (Table 1). From the scanning electron microscopic image of sample-1, zinc nanoparticles were observed to be spherical in shape with a size of 108.5 nm (Fig. 4).
FTIR Studies
The FTIR spectra were recorded for the Parthenium hysterophorous leaf extract (Fig. 5a) and sample-1 (Fig. 5b). The FTIR spectrum of Parthenium hysterophorous leaf extract showed the presence of functional groups of alcohols, phenols, alkenes, alkanes, carbonyls, aromatics, alkyl halides and alkynes. The spectrum showed the bands for the functional groups located at 3,779.57, 3,691.36, 2,910.73, 2,851.62, 1,672.48, 1,417.53, 1,275.29, 1,155.78 and 583.50 cm−1. The strong band of –C = O– stretch (carbonyls) was recorded at 1,672.48 cm−1. The medium bands of –C–C– (in ring) stretch (aromatics) and –CH2–X stretch (alkyl halides) were recorded at 1,417.53 and 1,155.78 cm−1. The FTIR spectrum of sample-1 showed the presence of functional groups of alcohols, phenols, alkenes, nitriles, carbonyls, aromatics, aliphatic amines, alkyl halides, alkenes and alkynes (Fig. 5). The IR spectrum of zinc nanoparticles showed the bands for the functional groups located at 3,673.15, 3,117.36, 2,388.24, 1,675.46, 1,410.28, 1,267.29, 1,162.84, 1,011.83, 800.57, 586.77 cm−1. The strong band of –C = O– stretch (carbonyls) was recorded at 1,675.46 cm−1. The medium bands of –C–C– (aromatics) and –CH2–X (alkyl halides) were recorded at 1,410.28, and 1,162.84 cm−1. From the FTIR spectra of Partheniumhysterophorous leaf extract and sample-1, the change in wave number of the functional groups was due to the reduction and stabilization of the metal group—Zn. When the spectrum of leaf extract was compared with the spectrum of sample-1, the functional groups of nitriles and aliphatic amines were observed from the spectrum of sample-1 due to the stretching of nitrile (C ≡ N) and amine (–NH2) bands.
Enzymatic activity
For the enzymatic activity analysis, acidic phosphatase activity, alkaline phosphatase activity and dehydrogenase activity were estimated for the pot culture, in which, sample-1 was added in three treatments, against the control in two regular time intervals of 30 and 60 days of sowing (Table 2). Sample-1 showed good results compared to sample-2. So sample-1 was taken for the enzyme and microbial activities and also to estimate the physiological parameters.
-
Treatment-1: 2.5 ml of sample-1 + 2.5 ml of distilled water.
-
Treatment-2: 1.0 ml of sample-1 + 1.0 ml of distilled water.
-
Treatment-3: 0.5 ml of sample-1 + 0.5 ml of distilled water.
-
Control: Without adding any sample.
The soil enzyme activities viz., dehydrogenase and phosphatase activities were performed after 30 and 60 days of sowing. The phosphatase and dehydrogenase enzyme activities were performed to test the enzymatic efficiency of the applied zinc nanoparticles. An overview of soil exo-enzymes is as follows—Soil phosphatase activity (Acid and Alkaline) was inhibited by the citrate phosphate buffer. The phosphatase activity was based on the determination of p-nitrophenol released after the incubation of oil with p-nitrophenol phosphate for 1 h at 37 °C. Acid and alkaline phosphatase activities differ only in terms of pH. Dehydrogenase activity was based on the estimation of the triphenyl tetrazolium chloride reduction rate to triphenyl formazan in soils after incubation at 30 °C for 24 h, where the tri phenyl tetrazolium is an artificial electron acceptor.
Enzyme activities were increased in response to all treatments when compared to the control confirmed that the zinc nanoparticles applied treatments showed significant variations in acid and alkaline phosphatase activity and dehydrogenase activity among the nanoparticles treatment after 30 and 60 days of sowing and also increased from 30 to 60 days time period against control when compared to Sunghyun et.al (2011). The measured phosphatases and dehydrogenases were cell bound and were not the extra-cellular activities. The slight raise in values for treatment-1 compared to treatment 2 and 3 is because sample-1 was applied in greater volume for treatment-1 (5 ml) compared to treatment-2 (2 ml) and treatment-3 (1 ml). The reason for performing the enzymatic activity analysis was to report that the applied zinc nanoparticles induce the phosphatase (acid and alkaline) and dehydrogenase enzyme activities of the pot culture experiment against the control. Our experiment revealed that the zinc nanoparticles induced the enzyme activity. Hence the green synthesized zinc nanoparticles were potential and non-toxic and also enhanced the phosphatase and dehydrogenase enzyme activities.
Microbial activity
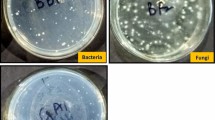
From the microbial population estimation, numbers of colonies were estimated for bacteria, fungi and actinomycetes for the three treatments (Treatment 1–3) along with the control. The results presented in Table 3 indicated that the bacterial population was significantly higher (7.2–7.4 × 105 g−1 dry soil) in nanoparticle-treated samples as compared to the control (5.5 × 105 g−1 dry soil). Similarly, the nanoparticle-treated samples had significantly higher fungi (2.0–2.4 × 103 g−1 dry soil) and actinomycetes (0.53–1.35 × 103 g−1 dry soil) compared to the controls. The microbial population estimation was performed to test the microbial activity in the soils to which zinc nanoparticles were applied. The results presented in Table 3 shows that the plant extracted zinc nanoparticles induce the rhizosphere microbial population. Compared to the control, treatments 1–3 showed higher number of colonies concluding that the applied zinc nanoparticles induced the growth of bacteria, fungi and actinomycetes. Among the three treatments, treatment-1 showed higher number of colonies as sample-1 is applied in greater volume for treatment-1. The reason for performing the microbial activity analysis was to report that the zinc nanoparticles induced the colony formation of microorganisms by applying the synthesized zinc nanoparticles to the bacterial, fungal and actinomycetes culture media by maintaining controls and incubating the petri-plates under controlled laboratory conditions. The microbial activity revealed that the synthesized zinc nanoparticles induce the growth of microbial population as the number of colonies were increased in response to all treatments, compared to the control.
Physiological traits
From the pot culture experiment, the physiological growth parameters—numbers of leaves, plant height, leaf surface ratio, root length, shoot length, fresh and dry weights of roots and shoots and total biomass were measured for the three treatments against the control. Leaf surface ratio, number of leaves and plant height were recorded after 30 and 60 days of sowing for all the three treatments against control.
Among the three treatments, treatment-1 showed high variations for all the physiological parameters against the control because for treatment-1, zinc nanoparticles (sample-1) were applied in greater volume compared to treatments 2 and 3. From Table 4, from control to treatment-1, leaf length was increased from 1.95 to 2.52 cm (increased by 22.6 %) after 30 days of sowing and from 2.25 to 2.73 cm (17.6 %) after 60 days of sowing. Leaf breadth increased from 1.02 to 1.24 cm (30 days, 17.7 %) and from 1.15 to 1.45 cm (60 days, 20.69 %). Number of leaves increased from 38 to 79 (30 days, 51.9 %) and from 46 to 98 (60 days, 53 %) and the plant height increased from 18.25 to 30.34 cm (30 days, 39.8 %) and from 30.14 to 50.41 cm (60 days, 40.21 %).
Simultaneously, root lengths and shoot lengths, fresh weight and dry weight of roots and shoots and total biomass were recorded for the three treatments against the control after 60 days of sowing (Table 5). Among the three treatments, treatment-1 showed high significant variations against the control. From control to treatment-1, root length increased from 1.92 to 7.29 cm (73.7 %), shoot length increased from 34.18 to 44.16 cm (22.6 %), fresh weight of root increased from 0.26 to 0.58 gm (55.2 %), dry weight of root increased from 0.13 to 0.23 gm (43.5 %), fresh weight of shoot increased from 4.81 to 13.33 gm (63.9 %), dry weight of shoot increased from 1.78 to 7.06 gm (74.8 %) and the total biomass increased from 1.91 to 7.29 gm (73.8 %) after 60 days of sowing.
The recorded physiological parameters concluded that the zinc nanoparticles applied plants showed better results when compared to the control. The results presented in Table 5 indicated that the effect of zinc nanoparticles on the growth of the Arachis hypogea L. pot culture was significantly high for treatment-1 than treatments 2–3 compared to the control after 30 and 60 days of sowing, and was also increased from 30 to 60 days time period of sowing. The results indicated that the effect of zinc nanoparticles on the root and shoot lengths, fresh and dry weight of roots and shoots and total biomass of the Arachis hypogea L. pot culture was also significantly high compared to the control after 60 days of sowing.
Conclusion
Green synthesis promises eco-friendly and non-toxic route to synthesize the zinc nanoparticles. This process can be easily scaled up to produce large quantities of zinc nanoparticles for nanobiotechnology industry and offers solutions to various problems in agriculture such as reducing the use of fertilizer, pesticide and water to improve plant breeding techniques. Here, the zinc nanoparticles were synthesized using Parthenium hysterophorous leaf extract by green synthesis route. The optical absorption peaks recorded at 327.50 and 330.00 nm confirms the formation of zinc nanoparticles. The estimated particle size was 16.10–58.60 nm, grain size was 108.50 nm and zeta potential was 100.40–117.20 mV, respectively. Sample-1 (100 %) exhibited highest zeta potential and least particle size. The enzyme and microbial activities and physiological traits were tested using sample-1, which showed significant variations among the nanoparticles treated samples compared to the control. The potential microbial activity of as prepared zinc nanoparticles is exciting. Zinc nanoparticles coupled with microbial activity promises potential applications in agriculture where zinc is one of the essential micronutrients which need to be supplied to the crop plants.
References
Badri Narayanan K, Sakthivel N (2008) Coriander Leaf mediated biosynthesis of gold nanoparticles. Mater Lett 62:4588–4590
Belz RG, Reinhardt CF, Foxcroft LC, Hurle K (2007) Residue allelopathy in Parthenium hysterophorus L.—does parthenin play a leading role? Crop Prot 26:237–245. doi:10.1016/j.cropro.2005.06.009
Ganesh Babu MM, Gunasekaran P (2009) Production and structural characterization of crystalline silver nanoparticles from Bacillus cereus isolate. Colloid Surface A 74:191–195
Gunalan S, Sivaraj Rajeshwari, Rajendran Venckatesh (2012) Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Progr Natur Sci 22:693–700
Kaushik N, Thakkar MS, Snehit S, Mhatre MS, Rasesh Y, Parikh MS (2010) Biological synthesis of metallic Nanoparticles. Nanomed Nanotechnol 6:257–262
Narayanan KB, Sakthive N (2010) Photosynthesis of gold nanoparticles using leaf extract of Coleus amboinicus Lour. Mater Charact 61:1232–1238
Oudhia P (2001) MEDICINAL Weeds in banana orchids: a boon for small farmers of chhattisgarh (INDIA). Agric. Sci. Digest 21:267–268
Philip Daizy (2010) Green synthesis of gold and silver nanoparticles using Hibiscus rosa sinensis. Phys E 42:1417–1424
Philip Daizy (2011) Mangifera indica leaf-assisted biosynthesis of well-dispersed silver nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc 78:327–331
Prasad TNVKV, Sudhakar P, Sreenivasulu Y, Latha P, Munaswamy V, Raja Reddy K, Sreeprasad TS, Sajanlal PR, Pradeep T (2012) Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J Plant Nutr 35:905–927
Revina AA, Oksentyuk EV, Fenin AA (2007) Synthesis and properties of zinc nanoparticles: the role and prospects of radiation chemistry in the development of modern nanotechnology. Prot Met 43:613–618. doi:10.1134/S0033173207060069
Sharma Nilesh C, Sahi Shivendra V, Nath Sudip, Parsons Jason G, Gardea-Torresdey Jorge L, Pal Tarasankar (2007) Synthesis of plant—mediated gold nanoparticles and catalytic role of bio matrix- embedded nanomaterials. Environ Sci Technol 41:5137–5142
Singh Ravindra P, Shukla Vineet K, Yadav Raghavendra S, Sharma Prashant K, Singh Prashant K, Pandey Avinash C (2011) Biological approach of zinc oxide nanoparticles formation and its characterization. Adv Mat Lett 2:313–317
Sunghyun Kim, Jungeun Kim, Insook Lee (2011) Effects of and ZnO nanoparticles and Zn2+ on soil enzyme activity and bioaccumulation of Zn in Cucumis sativus. Chem Ecol 27:49–55
Vinay Kumar B, Bhojya Naik HS, Girija D, Vijaya Kumar B (2011) ZnO nanoparticle as catalyst for efficient green one-pot synthesis of coumarins through Knoevenagel condensation. J Chem Sci 123:615–621
Wolf RE, Todd AS, Brinkman S, Lamothe PJ, Smith KS, Ranville JF (2009) Measurement of total Zn and Zn isotope ratios by quadrupole ICP-MS for evaluation of Zn uptake in gills of brown trout (Salmo trutta) and rainbow trout (Oncorhynchus mykiss). Talanta 80:676–684. doi:10.1016/j.talanta.2009.07.048
Zeng S, Yong K-T, Roy I, Dinh X-Q, Yu X, Luan F (2011) A review on functionalized gold nanoparticles for biosensing applications. Plasmonics 6:491. doi:10.1007/s11468-011-9228-1
Acknowledgments
Mrs. K. Sri Sindhura would like to thank Department of Science and Technology, New Delhi, India, for providing INSPIRE Fellowship and also Department of nanotechnology, Regional Agricultural Research Station (R.A.R.S), Acharya N. G Ranga Agricultural University, Tirupati and Indian Institute of Horticultural Research (IIHR, ICAR) for giving me permission to carry out the necessary laboratory work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Sri Sindhura, K., Prasad, T.N.V.K.V., Panner Selvam, P. et al. Synthesis, characterization and evaluation of effect of phytogenic zinc nanoparticles on soil exo-enzymes. Appl Nanosci 4, 819–827 (2014). https://doi.org/10.1007/s13204-013-0263-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-013-0263-4