Abstract
Introduction
IDegLira was shown to maintain glycemic control while reducing risk of hypoglycemia and body weight gain. The REX study was designed to generate real-world evidence on the use of IDegLira in Italian clinical practice in two different subgroups of patients, those switching to IDegLira from a basal insulin-supported oral therapy (BOT group) and those from a basal plus bolus insulin regimen (BB group).
Methods
Adult patients with T2D diagnosed for at least 12 months and having started IDegLira 2–3 months prior to enrolment, coming from a BOT or BB regimen, were enrolled in this multicenter observational prospective cohort study conducted in 28 Italian centers. This paper presents the methodological framework of the REX study and provides the interim analysis results describing the patients’ baseline characteristics and the clinical reasons for IDegLira treatment initiation.
Results
Of the 360 patients enrolled in the REX study, 331 were considered eligible for this interim analysis, 76.4% in the BOT and 23.6% in the BB group. Mean (SD) HbA1c was 8.5% (1.4) in the BOT and 8.2% (1.7) in the BB group. The most common T2D complications were diabetic macroangiopathy and diabetic nephropathy in both groups. The median (interquartile range) insulin daily dose before IDegLira was 15.0 (10.0–20.0) units in the BOT group and 42 (30.0–52.0) in the BB group. Oral antidiabetics were taken by 98% and 51.3% of patients, respectively. The main reason for switching to IDegLira was the inadequate glycemic control in the BOT group (86% of patients), and the intent to simplify the treatment in the BB group (66.7%).
Conclusions
IdegLira is initiated after BOT in inadequately controlled patients to improve glycemic control, whereas in BB patients it is used to simplify the therapeutic regimen. Final results of the REX study will shed light on patients’ outcomes after IdegLira treatment under routine clinical care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Patients with type 2 diabetes mellitus who do not achieve the target glycated hemoglobin (HbA1c) level despite escalating therapy may benefit from combinations of glucagon-like peptide 1 receptor agonists (GLP-1RAs) and basal insulin. |
The fixed-ratio combination of basal insulin degludec and the GLP-1RA liraglutide (IDegLira) was shown to maintain glycemic control while reducing risk of hypoglycemia and body weight gain. This allows simplification of multi-injection insulin schemes and reduction of oral therapies associated with basal insulin, as confirmed from both clinical trial and real-world evidence studies. |
The REX (Real-world Evidence Xultophy®) multicenter study is aimed at describing long-term glycemic control, alongside other relevant clinical parameters and treatment patterns associated with the use of IDegLira in a real-world clinical setting in Italy. |
REX study interim results provide valuable information on patient characteristics and show that IdegLira is initiated after a basal-supported oral therapy (BOT group) at a high baseline HbA1c mainly to improve glycemic control, whereas IdegLira is used in the basal plus bolus insulin regimen (BB group) mainly to simplify the therapeutic regimen. |
Introduction
Diabetes mellitus is a global, widespread, chronic condition that represents a major contributor to non-communicable diseases. The latest report of the International Diabetes Federation (IDF) estimated that nearly 537 million adults are diagnosed with diabetes mellitus (DM) worldwide [1], and this figure is expected to rise. According to the American Diabetes Association (ADA)/European Association for the Study of Diabetes (EASD), the goals of T2D treatment are to prevent or at least delay complications and maintain quality of life by controlling glycemia and managing cardiovascular risk factors, taking into account patient characteristics and preferences. Glycemic treatment targets should thus be individualized, as should the approach to therapy, on the basis of patient- and disease-related features [2].
Insulin treatment is usually initiated with basal insulin when patients do not reach or are no longer maintaining glycemic targets on non-insulin drugs. However, most patients treated with basal insulin still do not reach the target glycated hemoglobin (HbA1c) level. Generally, in these patients, clinicians intensify the insulin regimen by further uptitration of the basal insulin dose or with the addition of bolus insulin injections. Both these options increase the risk of hypoglycemia and weight gain. Recently, combinations of glucagon-like peptide 1 receptor agonists (GLP-1RAs) and basal insulin have been recommended by international guidelines as an alternative option. Such combinations have shown the same efficacy as the basal bolus treatment regimen in achieving glycemic control, but with a lower risk of hypoglycemia and weight gain and using a lower insulin dose [3,4,5].
IDegLira is a fixed-ratio combination of basal insulin degludec (IDeg) and the GLP-1RA liraglutide in a pre-filled pen for once-daily injection [6]. The efficacy and safety of IdegLira were established in the DUAL clinical trial program in patients with uncontrolled type 2 diabetes on oral antidiabetic drugs (DUAL I, IV, and VI) [7,8,9], GLP-1RAs (DUAL III) [10], basal insulin (DUAL II and V) [11, 12], and basal-bolus insulin (DUAL VII) [13]. Combination therapy with IDegLira was shown to reduce HbA1c more than monotherapy with liraglutide or insulin (degludec or glargine). Overall, the DUAL clinical trial program provided evidence of an advantage of IDegLira in maintaining glycemic control and reducing the risk of hypoglycemia and body weight gain.
IDegLira was approved in the European Union (EU) in September 2014 and in Italy in September 2017, with a recent label update allowing patients on any insulin regimen with a basal component, including the basal-bolus regimen, to switch to IDegLira. Several real-world evidence (RWE) studies have evaluated the effectiveness of IDegLira both as an intensification regimen in patients inadequately controlled by basal-supported oral therapy (BOT) and as a de-intensification regimen in patients on basal plus bolus insulin (BB) [14,15,16]. Real-world retrospective results from the European, multicenter EXTRA study suggest that patients switching from a BB regimen not only have the expected beneficial reductions in HbA1c and body weight after 6 months of treatment but also reduce their total daily insulin dose while avoiding the inconvenience of multiple dose injections [17]. Italian observational retrospective studies involving patients with T2D treated with IDegLira coming from either BOT or BB regimens with unsatisfactory glycemic control showed significant reductions in HbA1c and fasting blood glucose (FBG) with no gain in body weight and a lower number of concomitant diabetes medications [18,19,20,21]. In general, on the basis of randomized clinical trials and observational studies, between 50% and 60% of patients with T2D on BB can successfully stop bolus insulin by switching to a GLP-1RA, including the fixed-dose combination IdegLira [22, 23].
The REX (Real-world Evidence Xultophy®) multicenter study is aimed at describing the long-term glycemic control and other relevant clinical parameters and treatment patterns associated with the switch to IDegLira in patients with T2D coming from a BOT or a BB regimen in a real-world clinical setting in Italy. This paper presents the methodological framework of the REX study and provides the results of the interim analysis performed to describe the baseline clinical characteristics of patients at the time of IDegLira treatment initiation and the reasons for the choice of IDegLira treatment according to the different T2D management trajectories.
Methods
Study Design
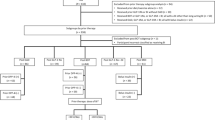
The REX study is an Italian multicenter observational prospective cohort study conducted in 28 centers across different Italian regions. This study involves both a primary data collection (during a prospective observation period of 18 months after enrolment visit) and secondary use of pre-existing data (during the retrospective observation period, from IDegLira initiation to enrolment visit). All participating patients received verbal and written information about the study and were given the opportunity to ask any questions to help them understand the study. They provided voluntary informed consent before data collection. This study was approved by the ethics committees of all participating institutions.
Patients were enrolled provided they had initiated IDegLira 2–3 months before enrolment and are then prospectively followed up for approximately 18 months, including those discontinuing treatment with IDegLira, unless consent is withdrawn. Data are collected during routine clinical visits, without additional diagnostic or monitoring procedures. The first patient in (FPI) was achieved on November 27, 2020. The study is expected to be completed in December 2022.
Study Population
The target population of the study consists of adult patients affected by T2D who started IDegLira according to the current clinical practice coming from a BOT or a BB regimen, with or without the addition of oral antidiabetics (OADs).
The main eligibility criteria include age 18 years or more, T2D diagnosed at least 12 months prior to enrolment treated with basal insulin ± OADs (BOT group) or with BB ± OADs (BB group) prior to initiating IDegLira, start of IDegLira at least 2 months but not more than 3 months before enrolment, and informed consent signature. The decision to initiate treatment with IDegLira had to be made by the patient and his/her treating physician before and independently from the decision to participate in the study. Patients on BB regimen are eligible provided that the bolus insulin component is stopped before IDegLira treatment initiation. Main exclusion criteria were diagnosis of type 1 diabetes, maturity-onset diabetes of the young, latent autoimmune diabetes in adults, gestational diabetes or any hyperglycemic state other than T2D.
A patient is considered withdrawn from the study if one of the following conditions applies: consent withdrawal, loss to follow-up, no available information at the last study visit, other reasons (e.g., pregnancy, death, study site closure).
Study Objectives
The purpose of the REX study is to describe long-term glycemic control alongside other relevant clinical parameters and treatment patterns associated with the use of IDegLira in a real-world clinical setting in Italy. The primary objective of the study is to evaluate the change in glycemic parameters after initiation of IDegLira in the study population. Secondary objectives are to describe the physician-provided rationale for initiating IDegLira, to evaluate changes in other clinical parameters of glycemic control and in treatment patterns associated with long-term IDegLira treatment, and to describe the outcomes of interest in the two distinct BOT and BB groups. The exploratory objective is to describe changes in further metabolic parameters of interest and in the number and type of concomitant T2D medications after IDegLira initiation.
Study Measures
The primary endpoint of the study is the change in HbA1c value from baseline to 6 months after IDegLira treatment initiation. Secondary endpoints to be evaluated from baseline to the end of observation are the change in HbA1c value beyond 6 months, the percentage of patients with HbA1c below 7%, the change in IDegLira daily dose, the number and severity of self-reported hypoglycemic episodes, and the percentage of patients with treatment intensification or simplification during observation; further variables and endpoints of interest are the reasons for switching to IDegLira (collected at enrolment but referred to the time of IDegLira initiation), the changes over time in specific clinical parameters (i.e., FBG, body weight, daily insulin dose, systolic/diastolic blood pressure, blood lipids, number and type of concomitant diabetes medication). All the aforementioned measures are evaluated overall in the study population, and separately in the BOT and BB groups.
Statistical Analysis
Sample Size
The sample size calculation was based on the primary endpoint, considering the need to reach a sufficient number of patients in the BB group switchers, which was expected to be less than the number of BOT patients (about 20% of participating patients). The sample size was estimated on the basis of an 80% probability of observing a mean (standard deviation, SD) HbA1c decrease of 0.6% (1.6) within 6 months of IDegLira treatment. This expected value applied to the overall study population, irrespective of treatment adherence or discontinuation during observation. At α = 0.05 and 80% power, the total number of patients to be enrolled in the study was estimated to be at least 338 (20% of whom possibly not evaluable for the primary analysis because of non-available HbA1c measurement at the 6-month follow-up visit).
Data Analysis
For the primary endpoint, and for some secondary and explorative endpoints, a mixed model for repeated measurements (MMRM) including all available measurements will be performed to evaluate the change in continuous parameters of interest from baseline over time. Both crude and adjusted estimates of the change in the analyzed variables will be presented, where adjusted estimate will be obtained by including in the model potential covariates, which are believed to have an influence on the change in each considered parameter (e.g., age, gender, corresponding baseline value, BMI classes, concomitant diabetes medications, treatment regimen prior to switch, diabetes duration, time since first insulin-based T2D regimen, and medical history). Results from the statistical analyses will be presented by the estimated treatment effect, with two-sided 95% confidence intervals (CIs) and associated p values, corresponding to a two-sided test of significance under the null hypothesis of no change. The effect evaluated by statistical analysis will be the change from baseline to selected time points: 6, 12, and/or 18 months after treatment initiation. For all binary outcomes, generalized estimating equation (GEE) or mixed effects logistic regression will be used.
Among other secondary and explorative endpoints, continuous variables will be reported as mean (SD) and median (interquartile range, IQR), while categorical variables will be reported as number and percentage of cases and distribution of frequencies.
In addition, sensitivity analyses will be performed to assess the robustness of primary and some secondary and exploratory analyses, involving patients “on treatment” with IDegLira at the time points of interest after treatment initiation. This will allow one to evaluate the effect of switching to IDegLira if all patients had adhered to treatment during follow-up and to characterize patients under continued treatment with IDegLira.
An interim analysis was conducted to evaluate the baseline patient characteristics and the clinical reasons for IDegLira treatment initiation once enrolment had been completed (Last Patient First Visit achieved on June 30, 2021). Results of this descriptive analysis are reported below. Descriptive analyses will be composed of means (SD), median (IQR), absolute and relative frequencies, according to the type of considered variables. No formal statistical hypotheses were set for this analysis.
Statistical analyses were performed using SAS Enterprise Guide v. 7.1 and SAS 9.4 (SAS Institute, Cary, NC, USA). Electronic case report form (eCRF) setup and statistical analyses were performed by MediNeos S.U.R.L. (Modena, Italy), a company subject to the direction and coordination of IQVIA Ltd, on behalf of Novo Nordisk.
Results
Study Population Demographics and Baseline Clinical Characteristics
A total of 360 patients were enrolled during a 7-month enrolment period, reaching the target sample size. Eighteen patients were excluded because of protocol violations and in 11 cases eligibility could not be verified at this stage, so 331 patients were considered eligible for this interim analysis.
At the time of IDegLira initiation, 76.4% (N = 253) of patients were on a basal insulin regimen, with or without the addition of OADs (BOT group) and 23.6% (N = 78) were on a basal-bolus insulin regimen, with or without OADs (BB group). Demographics and main baseline clinical characteristics divided by study group and in the overall eligible population are reported in Table 1.
BOT Group
In the BOT group, male patients accounted for 61.3% (N = 155) and mean (SD) age was 67.2 (10.0) years. On the basis of available BMI data, 38.2% (N = 66) of patients were overweight, and additional 46.8% (N = 81) were obese. At the time of IDegLira initiation (baseline) mean (SD) FBG level was 9.1 (3.3) mmol/L and mean (SD) HbA1c level was 8.5% (1.4). Concomitant conditions were reported at baseline in 81% (N = 205) of patients, mainly hypertension (74.1%) and dyslipidemia (61.5%). Mean (SD) age at T2D diagnosis was 51.7 (10.9) years and median (IQR) duration of the disease from the first diagnosis to the enrolment in the study was 14.2 (8.3–20.8) years. According to the patients’ medical history, the most common T2D complications present at the time of IDegLira treatment initiation were diabetic macroangiopathy (24.5% of patients) and diabetic nephropathy (18.6% of patients; details in Table 1).
BB Group
In the BB group, 47.4% of patients were male (N = 37) and mean (SD) age was 67.4 (10.5) years. Six patients (12.5%) had normal weight, 13 (27.1%) were overweight, and 29 (60.4%) were obese. At baseline mean (SD) FBG was 9.4 (3.2) mmol/L and mean (SD) HbA1c level was 8.2% (1.7). Concomitant conditions were present in 92.3% (N = 72) of patients, mainly hypertension (73.6%) and dyslipidemia (59.7%). Mean (SD) age at T2D diagnosis was 50.3 (12.1) years and median (IQR) duration of the disease from the first diagnosis to the enrolment in the study was 16.1 (11.1–21.1) years. Also in the BB group, the most common T2D complications were diabetic macroangiopathy (33.3% of patients) and diabetic nephropathy (29.5% of patients; details in Table 1).
Previous Diabetes Treatments
BOT Group
In the BOT group, the median (IQR) prescribed insulin daily dose before IDegLira initiation was 15.0 (10.0–20.0) units. The most used basal insulins were glargine U100 (49.2%, N = 124) and glargine U300 (29.8%, N = 75). The pre-study diabetes treatment regimen included OADs in 98% (N = 248) of BOT patients, mainly metformin, 79.8% (N = 202). Diabetes treatments administered prior to IDegLira initiation are detailed in Table 2, by study group and in the overall population. Combinations of therapies prior to IDegLira treatment initiation among BOT patients are depicted in Fig. 1. The median (IQR) time from the initiation of the first diabetes treatment regimen including insulin to the start of IDegLira was 29.1 (10.5–60.0) months.
BB Group
The median (IQR) prescribed insulin total daily dose before IDegLira was 42.0 (30.0–52.0) units in the BB group, including a median (IQR) dose of 18 (14.0–25.0) units of basal and 20 (14.0–27.0) units of bolus insulin. The most used basal insulins were glargine U100 (50.6%, N = 39) and glargine U300 (26.0%, N = 20). The most prescribed bolus insulins were lispro (45.3%, N = 34) and aspart (44%, N = 33). In the BB group, OADs were taken by 51.3% (N = 40) of BB patients, mainly metformin (43.6%, N = 34; Table 2). The median (IQR) time from the first insulin-including diabetes treatment to the start of IDegLira was 37.2 (12.5–106.3) months.
IDegLira Treatment Choice
BOT Group
In the BOT study subgroup, the main physician-provided reason for switching to IDegLira was the inadequate glycemic control obtained with the former T2D regimen (86% of patients, N = 218). The clinical reasons for switching to IDegLira are detailed in Table 3. The median (IQR) initial dosage of IDegLira was 16 (16.0–20.0) dose steps, corresponding to 16 units of insulin degludec and 0.6 mg of liraglutide.
BB Group
The main physician-provided reason for switching to IDegLira among BB patients was the intent to simplify the treatment regimen (66.7% of patients, N = 46; Table 3). The median (IQR) initial dosage of IDegLira was 16 (15.0–20.0) dose steps.
Discussion
The REX study has been designed to explore usage and effectiveness of IdegLira among insulin-treated individuals with T2D in real-world clinical practice in Italy. The protocol includes a baseline examination with retrospective data collection from each participant’s electronic medical records. Baseline data herein presented provide an opportunity to examine clinical characteristics of patients who initiated IdegLira and the reasons to initiate such treatment.
Of particular interest is the description of patients who initiated IdegLira to intensify a BOT regimen and patients who initiated IdegLira to de-intensify a BB regimen. Modern algorithms for the pharmacologic management of T2D recommend use of GLP-1RA before insulin in most patients [2], because GLP-1RAs grant greater or equal glycemic effects, significant weight loss, no hypoglycemia risk, and additional cardiorenal protection [24]. Furthermore, when a BOT regimen needs intensification, starting a GLP-1RA in either fixed of loose combination with BI, provides non-inferior glycemic control with much less weight and dramatically lower hypoglycemia rates [25]. Therefore, initiating IdegLira in people with T2D who were previously on BOT or BB insulin is rational and supported by available evidence and current recommendations. As expected, patients on BB insulin had a longer diabetes duration and more frequent chronic diabetic complications compared to patients on BOT. This clearly suggests that patients on BB insulin were, as expected, in a later disease stage than those on BOT. Yet, patients on BB insulin had a lower baseline HbA1c (mean 8.2% and 8.5%, respectively). On the one hand, this observation highlights once more that intensification of a BOT insulin regimen is pursued with a significant delay and a substantial hyperglycemic burden as compared to the recommended standards of care. On the other hand, initiation of IdegLira among patients on BB insulin at a lower HbA1c is consistent with the aim of simplifying the treatment regimen. Indeed, the most commonly reported reason to initiate IdegLira after BOT was inadequate glycemic control, whereas the most common reason to initiate IdegLira among patients on BB was the intent to simplify therapy. According to the IdegLira reimbursement criteria in Italy, bolus insulin has to be withdrawn at the time of IdegLira initiation. Therefore, this therapeutic trajectory truly represents a treatment simplification, as patients will experience a substantial reduction in the daily number of injections and glucose checks, with possible improvement in body weight, and a lower risk of hypoglycemia as compared to the previous regimen. In addition, the baseline characteristics of overall patients included in the REX study, showing multiple risk factors and associated comorbidities, could also underlie the opportunity to introduce a treatment known to have a cardiovascular benefit [11, 13, 26,27,28,29]. From a post hoc analysis of the phase III clinical studies included in the DUAL clinical development program, it emerged that, compared with insulin comparators, treatment with IDegLira was associated with significant reduction in HbA1c (DUAL II and V) and body weight; a greater decrease in systolic blood pressure (DUAL VII); a small but statistically significant increase in mean heart rate (DUAL VII); reduced levels of total cholesterol (DUAL II, V, and VII), LDL cholesterol (DUAL II and V), and HDL cholesterol (DUAL VII); reduced levels of free fatty acids (DUAL V, VII, and VIII), apolipoprotein B and brain natriuretic peptide (BNP) (DUAL II) [11,12,13, 29, 30].
Prior studies have demonstrated that addition of GLP-1RA allows a stable withdrawal of bolus insulin in 50–60% of patients, especially those with a relatively short disease duration, better glycemic control, and lower insulin requirements [31]. The observational follow-up part of the REX protocol will assess effectiveness and persistence of the therapeutic effects over time after initiating IdegLira in both the BOT and BB groups. In this regard, some key features of the REX study protocol deserve to be highlighted. Differently from most other available observational studies on IdegLira and other glucose-lowering medications, the REX study will collect follow-up data in a prospective way. Though more resource-demanding and time-consuming, this methodological approach has several important advantages over retrospective data surveys from electronic medical records. First, several safety outcomes, including hypoglycemia, can be collected much more reliably when patients are actively enrolled in a prospective study as compared to what occurs with retrospective series. Second, prospective evaluation allows collection of information on hypoglycemia events, treatment simplification and daily insulin dose reduction, with a strong impact on the patient’s quality of life. Duration of observation is another key feature of the REX study, because patients will be prospectively followed for 18 months. This is substantially longer than the duration of most prior studies on IdegLira and other glucose-lowering medications, including GLP-1RA and some real-world studies assessing cardiovascular outcomes of diabetes drugs [32,33,34].
As this is a non-interventional study, potential selection bias such as confounding may be present, which may warrant further exploration. Data collection reflects routine clinical practice rather than mandatory assessments at pre-specified time points, which may have an impact on the amount of data and their interpretation. In particular, the partially retrospective data collection may imply some inhomogeneity in the availability and quality of data. For example, though most physicians were using the same electronic chart and coding systems, there was no standardization on the definition and diagnosis of diabetic complications. The lack of a control group of patients not receiving IDegLira is another limitation that makes results of this observational study purely descriptive. Furthermore, this article refers to an interim analysis limited to the baseline demographics and clinical characteristics of enrolled patients, only allowing one to preliminarily explore the representativeness of the population. Yet, we believe it is of interest to provide an initial insight into which types of patients and for what reasons IDegLira is chosen in Italian clinical practice.
Conclusions
This interim analysis of the REX study provides valuable information on the usage pattern of IdegLira in Italian clinical practice which appears to reflect the potential benefits expected by diabetologists in two different populations of individuals with T2D, those previously treated with either BOT or BB insulin. IdegLira is initiated after BOT in patients with high baseline HbA1c mainly to improve glycemic control and in patients on a BB insulin regimen mainly to simplify the therapeutic regimen. The final results of the ongoing prospective evaluation will shed light on the long-term patient outcomes after IdegLira initiation under routine clinical care.
References
International Diabetes Federation. IDF Diabetes Atlas. 10th edition 2021. https://www.diabetesatlas.org. Accessed 11 Apr 2022.
Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669–701.
Anderson SL, Trujillo JM. Basal insulin use with GLP-1 receptor agonists. Diabetes Spectr. 2016;29:152–60.
Castellana M, Cignarelli A, Brescia F, Laviola L, Giorgino F. GLP-1 receptor agonist added to insulin versus basal-plus or basal-bolus insulin therapy in type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2019;35(1): e3082.
Fadini GP, Disoteo O, Candido R, et al. Delphi-based consensus on treatment intensification in type 2 diabetes subjects failing basal insulin supported oral treatment: focus on basal insulin + GLP-1 receptor agonist combination therapies. Diabetes Ther. 2021;12(3):781–800.
XULTOPHY® (IDegLira) Summary of product characteristics, updated on 24/09/2020. https://www.ema.europa.eu/documents/product-information/xultophy-epar-product-information_en.pdf. Accessed 11 Apr 2022.
Gough SC, Bode B, Woo V, et al. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2:885–93.
Rodbard HW, Bode BW, Harris SB, et al. Safety and efficacy of insulin degludec/liraglutide (IDegLira) added to sulphonylurea alone or to sulphonylurea and metformin in insulin-naïve people with type 2 diabetes: the DUAL IV trial. Diabet Med. 2017;34:189–96.
Harris SB, Kocsis G, Prager R, et al. Safety and efficacy of IDegLira titrated once weekly versus twice weekly in patients with type 2 diabetes uncontrolled on oral antidiabetic drugs: DUAL VI randomized clinical trial. Diabet Obes Metab. 2017;19:858–65.
Linjawi S, Bode BW, Chaykin LB, et al. The efficacy of IDegLira (insulin degludec/liraglutide combination) in adults with type 2 diabetes inadequately controlled with a GLP-1 receptor agonist and oral therapy: DUAL III randomized clinical trial. Diabet Ther. 2017;8:101–14.
Buse JB, Vilsbøll T, Thurman J, et al. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabet Care. 2014;37:2926–33.
Lingvay I, Pérez Manghi F, Garcìa-Hernàndez P, et al. DUAL V Investigators. Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized clinical trial [published correction appears in JAMA 2016;315:2125]. JAMA. 2016;315:898–907.
Billings LK, Doshi A, Gouet D, et al. Efficacy and safety of IDegLira versus basal-bolus insulin therapy in patients with type 2 diabetes uncontrolled on metformin and basal insulin: the DUAL VII randomized clinical trial. Diabetes Care. 2018;41(5):1009–16.
Drummond R, Baru A, Dutkiewicz M, Basse A, Tengmark BO. Physicians’ real-world experience with IDegLira: results of a European survey. BMJ Open Diabet Res Care. 2018;6(1): e000531.
Melzer-Cohen C, Chodick G, Naftelberg S, Shehadeh N, Karasik A. Metabolic control and adherence to therapy in type 2 diabetes mellitus patients using IDegLira in a real-world setting. Diabet Ther. 2020;11(1):185–96.
Egede LE, Bogdanov A, Fischer L, et al. Glycemic control among patients newly prescribed IDegLira across prior therapy group in us real-world practice. Diabetes Ther. 2020;11(7):1579–89.
Price H, Blüher M, Prager R, et al. Use and effectiveness of a fixed-ratio combination of insulin degludec/liraglutide (IDegLira) in a real-world population with type 2 diabetes: results from a European, multicentre, retrospective chart review study. Diabet Obes Metab. 2018;20(4):954–62.
Di Loreto C, Celleno R, Piastrella L, Del Sindaco P. IDegLira fixed-ratio combination in the real world: a retrospective observational single-center Italian experience. Eur Rev Med Pharmacol Sci. 2020;24(20):10671–9.
Zenari L, Da Porto A, De Moliner L, et al. Real-world evaluation of glycemic outcomes and extra-glycemic parameters in diabetic patients treated with the combined formulation degludec-liraglutide (Ideglira). Diabet Ther. 2021;12(1):197–209.
Persano M, Nollino L, Sambataro M, et al. Real-world study on the effectiveness and safety of basal insulin IDegLira in type 2 diabetic patients previously treated with multi-injective insulin therapy. Eur Rev Med Pharmacol Sci. 2021;25(2):923–31.
Rizza S, Piciucchi G, Mavilio M, et al. Effect of deprescribing in elderly patients with type 2 diabetes: iDegLira might improve quality of life. Biomed Pharmacother. 2021;144: 112341.
Rosenstock J, Nino A, Soffer J, et al. Impact of a weekly glucagon-like peptide 1 receptor agonist, albiglutide, on glycemic control and on reducing prandial insulin use in type 2 diabetes inadequately controlled on multiple insulin therapy: a randomized trial. Diabet Care. 2020;43(10):2509–18.
Bonora BM, Rigato M, Frison V, et al. Deintensification of basal-bolus insulin after initiation of GLP-1RA in patients with type 2 diabetes under routine care. Diabet Res Clin Pract. 2021;173: 108686.
Abd El Aziz MS, Kahle M, Meier JJ, Nauck MA. A meta-analysis comparing clinical effects of short- or long-acting GLP-1 receptor agonists versus insulin treatment from head-to-head studies in type 2 diabetic patients. Diabetes Obes Metab. 2017;19(2):216–27.
Maiorino MI, Chiodini P, Bellastella G, et al. Free and fixed-ratio combinations of basal insulin and GLP-1 receptor agonists versus basal insulin intensification in type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabet Obes Metab. 2018;20(9):2309–13.
Marso SP, McGuire DK, Zinman B, et al. Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med. 2017;377(8):723–32.
Tack C, Jacob S, Desouza C, et al. Liraglutide effects in insulin-treated patients in LEADER. Diabetes 2018;67(null):A117.
Brown-Frandsen K, Emerson SS, Marso SP, et al. Rates of major adverse cardiovascular (CV) events (MACE) and mortality with basal insulin by liraglutide use-a DEVOTE subanalysis. Diabetes 2018;67(null):A281–A282.
Vilsbøll T, Blevins TC, Jodar E, et al. Fixed-ratio combination of insulin degludec and liraglutide (IDegLira) improves cardiovascular risk markers in patients with type 2 diabetes uncontrolled on basal insulin. Diabet Obes Metab. 2019;21(6):1506–12.
Aroda VR, González-Galvez G, Grøn R, et al. Durability of insulin degludec plus liraglutide versus insulin glargine U100 as initial injectable therapy in type 2 diabetes (DUAL VIII): a multicentre, open-label, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(8):596–605.
Giorgino F, Bonadonna RC, Gentile S, Vettor R, Pozzilli P. Treatment intensification in patients with inadequate glycemic control on basal insulin: rationale and clinical evidence for the use of short-acting and other glucagon-like peptide-1 receptor agonists. Diabet Metab Res Rev. 2016;32(6):497–511.
Morieri ML, Consoli A, Sesti G, et al. Comparative effectiveness of dapagliflozin vs DPP-4 inhibitors on a composite endpoint of HbA1c, body weight and blood pressure reduction in the real world. Diabetes Metab Res Rev. 2021;37(1):e3353.
Fadini GP, Bonora BM, Lapolla A, et al. Comparative effectiveness of exenatide once-weekly versus liraglutide in routine clinical practice: a retrospective multicentre study and meta-analysis of observational studies. Diabetes Obes Metab. 2019;21(5):1255–60.
Patorno E, Pawar A, Franklin JM, et al. Empagliflozin and the risk of heart failure hospitalization in routine clinical care. Circulation. 2019;139(25):2822–30.
Acknowledgements
We are very grateful to all patients participating in this study. We also wish to extend our grateful thanks to MediNeos—a company subject to the direction and coordination of IQVIA Ltd—for their contribution in the REX study conduction, scientific support, clinical operations, data management and statistical analysis (Alessandra Ori, Alessandro Zullo, Barbara Roncari, Daniele Andreis, Beatrice Grossi, Saide Sala, Francesca Trevisan), and to Renata Perego for her editorial assistance in the preparation of the manuscript.
Funding
This study and journal’s Rapid Service Fee have been funded by Novo Nordisk.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
G. Lastoria and L. Simoni contributed to the conception and design of the study. In addition, L. Simoni contributed to the data analysis. G.P. Fadini, R. Buzzetti, M.R. Fittipaldi, F. D’Incau, A. Da Porto, A. Girelli and A. Consoli participated in the acquisition of data and interpretation of results. G.P. Fadini, G. Lastoria and L. Simoni wrote the manuscript draft; R. Buzzetti, M.R. Fittipaldi, F. D’Incau, A. Da Porto, A. Girelli and A. Consoli revised it critically for important intellectual content. All authors read and approved the final manuscript.
Investigators
REX study group includes the investigators involved in the study: Nicolangelo Iazzetta, Giuseppe Di Giovanni, Ornella Carbonara (Naples); Concetta Aragiusto, Diego Carleo, Nicoletta Da Rosa (Casoria, Naples); Emilia Martedì (Portici, Naples); Luigi Landolfi, Marta Marracino, Anna Tortora (Salerno); Gianluca De Morelli (Praia a Mare, Cosenza); Viviana Casarsa (Udine); Ernesto Maddaloni, Antonio Siena (Rome); Dario Pitocco, Linda Tartaglione, Alessandro Rizzi (Rome); Frida Leonetti, Martina Fasolo, Gabriele Morsello (Latina); Rocco Bulzomì, Gilda Ruga, Andrea Bianconi (Rome); Enrico Torre; Alberto Rebora, Francesca Cecoli, Eleonora Monti (Genoa); Silvia Bonfadini, Silvia Dotti, Sara Madaschi (Brescia); Roberto Trevisan, Mascia Albizzi, Rosalia Bellante, Anna Corsi, Cristina Scaranna (Bergamo); Pasquale De Cata, Federico Liboà, Stefania Ghilotti (Pavia); Elena Tortato, Luigi Lanari, Federica Turchi (Ancona); Enrico Gabellieri (Alessandria); Olga Lamacchia, Cinzia Colucci (Foggia); Giovanni Mileti (Francavilla Fontana, Brindisi); Sara Coluzzi, Federica Carrieri (Chieti/Pescara); Paola Rossetti, Massimiliano Anzaldi, Catania; Antonino Di Benedetto; Domenica Ruggeri (Messina); Alessia Scatena, Anna Ranchelli, Ivana Ragusa (Arezzo); Giovanna Gregori, Isabella Crisci, Mary Mori, Fabio Baccetti (Carrara, Massa-Carrara); Roberto Anichini, Elisabetta Salutini (Pistoia); Carmela Vinci, Isabella Colletti, Milena Sira Zanon (San Donà di Piave, Venice); Anna Altomari (Feltre, Belluno); Benedetta Maria Bonora (Padua).
Disclosures
G.P. Fadini has received honoraria, grants or lecture fees from Abbott, AstraZeneca, Boehringer, Lilly, MSD, Novartis, Novo Nordisk, Mundipharma, Sanofi, Servier, Takeda. R. Buzzetti has received advisory board and speaker honoraria from Novo Nordisk, Sanofi, Eli Lilly, Abbott and speaker honoraria from AstraZeneca, Mundipharma. F. D’Incau has received a speaker honorarium from Novo Nordisk. A. Da Porto has received funding for attending advisory boards from Mundipharma, Boehringer Ingelheim and Novo Nordisk; and has received speaker fees and honoraria from Boehringer Ingelheim, Lilly, Mundipharma, AstraZeneca, Novo Nordisk, Sanofi-Aventis and Takeda. A. Consoli declares that in the past 5 years he has received speaker’s fee from Abbot, AstraZeneca, Boheringer Ingelheim, Eli Lilly, MSD, Menarini Diagnostici, Novo Nordisk, Sanofi-Aventis, Sigma-Tau, Takeda; has participated in Advisory Board Meetings of AstraZeneca, Boheringer Ingelhaim, Eli Lilly, Janssen Farmaceutici, MSD, Novo Nordisk, Sanofi-Aventis; has served as consultant for AstraZeneca and Novo Nordisk; and has received Research Grants from AstraZeneca, Eli Lilly and Novo Nordisk. G. Lastoria is an employee of Novo Nordisk Italy (Medical Affairs Department). L. Simoni is employee of MediNeos Observational Research (an IQVIA company, Italy), hired by Novo Nordisk, responsible for the conduction of the REX study, as well as scientific support, clinical operations, data management, statistical analysis. M.R. Fittipaldi and A. Girelli declare that they have no conflict of interest.
Compliance with Ethics Guidelines
All participating patients received verbal and written information about the study and were given the opportunity to ask any questions to help them understand the study. They provided voluntary informed consent before data collection. The REX study was approved by the ethics committees of all participating institutions. In particular, the study was firstly submitted to the “Comitato Etico Campania Sud” Ethics Committee of the San Francesco d’Assisi Hospital (Oliveto Citra, Salerno) and received approval on 02 October 2020. This study is being conducted in accordance with the Helsinki Declaration of 1964, and its later amendments.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to local legal restriction and regulations but are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
The members of REX study group are listed in Acknowledgments.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Fadini, G.P., Buzzetti, R., Fittipaldi, M.R. et al. IDegLira for the Real-World Treatment of Type 2 Diabetes in Italy: Protocol and Interim Results from the REX Observational Study. Diabetes Ther 13, 1483–1497 (2022). https://doi.org/10.1007/s13300-022-01287-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-022-01287-z