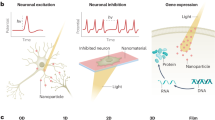
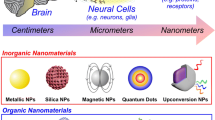
Abstract
Neuromodulation, as a fast-growing technique in neuroscience, has been a great tool in investigation of the neural pathways and treatments for various neurological disorders. However, the limitations such as constricted penetration depth, low temporal resolution and low spatial resolution hindered the development and clinical application of this technique. Nanotechnology, which refers to the technology that deals with dimension under 100 nm, has greatly influenced the direction of scientific researches within recent years. With the recent advancements in nanotechnology, much attention is being given at applying nanomaterials to address the limitations of the current available techniques in the field of biomedical science including neuromodulation. This mini-review aims to introduce the current state-of-the-art stimuli-responsive nanomaterials used for assisting thermally induced neuromodulation.





Similar content being viewed by others
References
Johnson MD, Lim HH, Netoff TI, Connolly AT, Johnson N, Roy A, Holt A, Lim KO, Carey JR, Vitek JL, He B. Neuromodulation for brain disorders: challenges and opportunities. IEEE Trans Biomed Eng. 2013;60:610–24.
Wong KH, Riaz MK, Xie Y, Zhang X, Liu Q, Chen H, Bian Z, Chen X, Lu A, Yang Z. Review of current strategies for delivering Alzheimer’s disease drugs across the blood-brain barrier. Int J Mol Sci. 2019;20:381.
Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7:a020412–a020412.
Lozano AM, Lipsman N, Bergman H, Brown P, Chabardes S, Chang JW, Matthews K, McIntyre CC, Schlaepfer TE, Schulder M, Temel Y, Volkmann J, Krauss JK. Deep brain stimulation: current challenges and future directions. Nat Rev Neurol. 2019;15:148–60.
Polanía R, Nitsche MA, Ruff CC. Studying and modifying brain function with non-invasive brain stimulation. Nat Neurosci. 2018;21:174–87.
Paviolo C, Stoddart PR. Gold nanoparticles for modulating neuronal behavior. Nanomaterials. 2017;7:1–14.
Rizvi SMD, Hussain T, Ahmed ABF, Alshammari TM, Moin A, Ahmed MQ, Barreto GE, Kamal MA, Ashraf GM. Gold nanoparticles: a plausible tool to combat neurological bacterial infections in humans. Biomed Pharmacother. 2018;107:7–18.
Raliya R, Saha D, Chadha TS, Raman B, Biswas P. Non-invasive aerosol delivery and transport of gold nanoparticles to the brain. Sci Rep. 2017;7:1–8.
Paviolo C, Haycock JW, Cadusch PJ, McArthur SL, Stoddart PR. Laser exposure of gold nanorods can induce intracellular calcium transients. J Biophotonics. 2014;7:761–5.
Eom K, Kim J, Choi JM, Kang T, Chang JW, Byun KM, Jun SB, Kim SJ. Enhanced infrared neural stimulation using localized surface plasmon resonance of gold nanorods. Small. 2014;10:3853–7.
Yoo S, Hong S, Choi Y, Park JH, Nam Y. Photothermal inhibition of neural activity with near-infrared-sensitive nanotransducers. ACS Nano. 2014;8:8040–9.
Carvalho-de-Souza JL, Treger JS, Dang B, Kent SBH, Pepperberg DR, Bezanilla F. Photosensitivity of neurons enabled by cell-targeted gold nanoparticles. Neuron. 2015;86:207–17.
Lee JW, Jung H, Cho HH, Lee JH, Nam Y. Gold nanostar-mediated neural activity control using plasmonic photothermal effects. Biomaterials. 2018;153:59–69.
Kim HW, Yang K, Jeong WJ, Choi SJ, Lee JS, Cho AN, Chang GE, Cheong E, Cho SW, Lim YB. Photoactivation of noncovalently assembled peptide ligands on carbon nanotubes enables the dynamic regulation of stem cell differentiation. ACS Appl Mater Interfaces. 2016;8:26470–81.
Wallyn J, Anton N, Vandamme TF. Synthesis, principles, and properties of magnetite nanoparticles for in vivo imaging applications—A review. Pharmaceutics. 2019;11:1–29.
Chechetka SA, Yu Y, Zhen X, Pramanik M, Pu K, Miyako E. Light-driven liquid metal nanotransformers for biomedical theranostics. Nat Commun. 2017;8:1–19.
Lyu Y, Xie C, Chechetka SA, Miyako E, Pu K. Semiconducting polymer nanobioconjugates for targeted photothermal activation of neurons. J Am Chem Soc. 2016;138:9049–52.
Wu X, Jiang Y, Rommelfanger NJ, Yin R, Liu J. Through-scalp deep-brain stimulation in tether-free , naturally-behaving mice with widefield NIR-II illumination. bioRxiv. 2020; 1–35.
Li B, Wang Y, Gao D, Ren S, Li L, Li N, An H, Zhu T, Yang Y, Zhang H, Xing C. Photothermal modulation of depression-related ion channel function through conjugated polymer nanoparticles. Adv Funct Mater. 2021;2010757:1–9.
Maiti D, Tong X, Mou X, Yang K. Carbon-based nanomaterials for biomedical applications: a recent study. Front Pharmacol. 2019;9:1–16.
Miyako E, Russier J, Mauro M, Cebrian C, Yawo H, Ménard-Moyon C, Hutchison JA, Yudasaka M, Iijima S, De Cola L, Bianco A. Photofunctional nanomodulators for bioexcitation. Angew Chemie. 2014;126:13337–41.
Paviolo C, Haycock JW, Yong J, Yu A, Stoddart PR, Mcarthur SL. Laser exposure of gold nanorods can increase neuronal cell outgrowth. Biotechnol Bioeng. 2013;110:2277–91.
Huang H, Delikanli S, Zeng H, Ferkey DM, Pralle A. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat Nanotechnol. 2010;5:602–6.
Dordick S, Friedman JM. Plasma glucose in mice. 2013; 336:604–608.
Chen R, Romero G, Christiansen MG, Mohr A, Anikeeva P. Wireless magnetothermal deep brain stimulation. Science. 2015;347:1477–80.
Stanley SA, Kelly L, Latcha KN, Schmidt SF, Yu X, Nectow AR, Sauer J, Dordick JS, Friedman JM. Bidirectional electromagnetic control of the hypothalamus regulates feeding and metabolism. Nature. 2016;531:1–20.
Munshi R, Qadri SM, Pralle A. Transient magnetothermal neuronal silencing using the chloride channel anoctamin 1 (TMEM16A). Front Neurosci. 2018;12:1–13.
Munshi R, Qadri SM, Zhang Q, Rubio IC, del Pino P, Pralle A. Magnetothermal genetic deep brain stimulation of motor behaviors in awake, freely moving mice. Elife. 2017;6:1–26.
Rao S, Chen R, LaRocca AA, Christiansen MG, Senko AW, Shi CH, Chiang PH, Varnavides G, Xue J, Zhou Y, Park S, Ding R, Moon J, Feng G, Anikeeva P. Remotely controlled chemomagnetic modulation of targeted neural circuits. Nat Nanotechnol. 2019;14:967–73.
Rosenfeld D, Senko AW, Moon J, Yick I, Varnavides G, Gregureć D, Koehler F, Chiang PH, Christiansen MG, Maeng LY, Widge AS, Anikeeva P. Transgene-free remote magnetothermal regulation of adrenal hormones. Sci Adv. 2020;6:1–12.
Acknowledgements
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF-2020R1C1C1007589), Korea Medical Device Development Fund grant funded by the Korean government (Ministry of Science and ICT, Ministry of Trade, Industry and Energy, Ministry of Health & Welfare, Ministry of Food and Drug Safety) (NTIS no. 9991006805), National R&D Program through the National Research Foundation of Korea (NRF) funded by Ministry of Science and ICT (2021M3H4A1A03048648/2021M3F3A2A01037365), the Smart Project Program through KAIST–Khalifa Joint Research Center (KK-JRC), KAIST College of Engineering Global Initiative Convergence Research Program, KI Meta Convergence Research Program, and KAIST Post-AI Research Project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Yang C declares that s/he has no conflict of interest in relation to the work in this article. Park S declares that s/he has no conflict of interest in relation to the work in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, C., Park, S. Nanomaterials-assisted thermally induced neuromodulation. Biomed. Eng. Lett. 11, 163–170 (2021). https://doi.org/10.1007/s13534-021-00193-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-021-00193-w