Abstract
Numerous cell types are involved in maintenance of the intestinal tissue. However, the main players are cells of the epithelial lining and the immune system. Human peripheral blood mononuclear cells (PBMCs) are used to investigate the effect of food bioactives on various immune cells. These cells are easily isolated from blood of healthy donors or buffy coats (leukocyte concentrates, a by-product from hospital Blood Banks in the manufacturing of red blood cell and thrombocyte concentrates from anti-coagulated whole blood). PBMCs have a different composition, phenotype and activation status than cells found in intestinal tissue. However, this is a useful test system for investigation of immune modulatory effects of food bioactive compounds. Methods for the isolation of PBMCs and how they are used to investigate effects of bioactive components are discussed in this chapter.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Origin
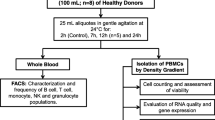
Human peripheral blood mononuclear cells (PBMCs) are isolated from peripheral blood and identified as any blood cell with a round nucleus (i.e. lymphocytes, monocytes, natural killer cells (NK cells) or dendritic cells). The cell fraction corresponding to red blood cells and granulocytes (neutrophils, basophils and eosinophils) is removed from whole blood by density gradient centrifugation. A gradient medium with a density of 1.077 g/ml separates whole blood into two fractions; PBMCs makes up the population of cells that remain in the low density fraction (upper fraction), whilst red blood cells and PMNs have a higher density and are found in the lower fraction (Fig. 15.1).
PBMCs include lymphocytes (T cells, B cells, and NK cells), monocytes, and dendritic cells. In humans, the frequencies of these populations vary across individuals, but typically, lymphocytes are in the range of 70–90 %, monocytes from 10 to 20 %, while dendritic cells are rare, accounting for only 1–2 %. The frequencies of cell types within the lymphocyte population include 70–85 % CD3+ T cells, 5–10 % B cells, and 5–20 % NK cells. The CD3+ lymphocytes are composed of CD4+ and CD8+ T cells, roughly in a 2:1 ratio. After activation the CD4+ T cell subset may develop into diverse effector cell subsets, including Th1, Th2, Th17, Th9, Th22, follicular helper (Tfh) cells and different types of regulatory cells (Akdis et al. 2012; Crotty 2011; Tan and Gery 2012; Sakaguchi et al. 2008). The CD4+ helper T cells are essential mediators of immune homeostasis and inflammation (Hirahara et al. 2013).
2 Features and Mechanisms
Most of the PBMCs are naïve or resting cells without effector functions. In the absence of an ongoing immune response T cells, the largest fraction of the isolated PBMCs, are mainly present as naïve or memory T cells. The naïve T-cells have never encountered their cognate antigen before and are commonly characterized by the absence of activation markers like CD25, CD44 or CD69 and the absence of the memory marker CD45RO isoform. Antigen recognition by a naïve T cell may result in activation of the cell (described in detail in Chap. 18), which will then enter a differentiation program and develop effector functions. Activated T cells may further differentiate into memory T cells that are able to mount a faster and stronger immune response the second time the antigen is encountered. B cells are also present in blood as naïve cells with a surface-anchored antigen receptor. Upon encountering antigen, the B cell may become activated and differentiate into antibody-producing plasma cells.
In peripheral blood the frequency of lymphocytes with specificity for a single antigen are low. Polyclonal activators are therefore used for in vitro stimulation as these will activate a large proportion of the lymphocytes, independently of their antigen specificity. The most common activators are mitogenic lectins, carbohydrate-binding proteins that bind to a number of glycoproteins expressed on the plasma membrane of lymphocytes (Ashraf and Khan 2003). Also, polyclonal activation of T cells can be obtained by antibodies that specifically bind to CD3, alone or in combination with CD28. The most commonly used mitogenic lectins are phytohaemagglutinin (PHA) and concanavalin A (con A) that mainly induce T cell proliferation, pokeweed mitogen (PWM) that induce T and B cell proliferation and lipopolysaccharide (LPS) which induce B cell proliferation and activation of monocytes. Effects on the immune function of PBMCs are usually investigated by measuring changes in lymphocyte proliferation, characterization of cytokine secretion profiles or changes in gene expression.
3 Stability, Consistency and Reproducibility
It is well known that multiple physiological factors such as nutritional status, hormone levels and infections/inflammation will influence the reactivity of immune cells; hence, the composition of PBMCs will depend on donor and the donor’s physiological status. Compared to the use of cell lines, this can lead to increased inter-experimental variation when different donors are used. Although the use of different donors leads to increased variance, the strength of reproducing results with cells from several donors will support the generality of the results.
4 Relevance to Human In Vivo Situation
PBMCs isolated from whole blood will be different from immune cells isolated from intestinal tissue or lymph nodes draining the intestine. Intraepithelial lymphocytes are predominantly CD8+ (90 %) cells whilst lymphocytes within the lamina propria are mainly CD4+ cells expressing a CD44hiCD62L− effector memory phenotype which indicate that these are antigen-experienced cells (Shale et al. 2013). In addition, several populations of immune cells present in the intestinal mucosa is not present in blood i.e. B1 B cells, natural killer T cells (NKT cells) and innate lymphoid cells (ILCs). Upon inflammatory signals, monocytes from blood will move into the site of infection and differentiate to myeloid antigen-presenting cells which is a heterogeneous population of macrophages and dendritic cells (DCs) (Swiatczak and Rescigno 2012). Because of these differences in the phenotype of cells present in blood and in lamina propria of the gastrointestinal tract it can be expected that mononuclear cells isolated from blood will respond differently from lamina propria mononuclear cells. Care should therefore be taken in drawing conclusions about the mucosal immune response in the intestine from in vitro studies with PBMCs.
5 General Protocol
Isolation of PBMCs is done from peripheral blood or buffy coats supplemented with anticoagulants (heparin, EDTA, citrate, ACD-A or citrate phosphate dextrose (CPD)). Blood is diluted with 2–4 volumes phosphate-buffered saline (PBS) pH-7.2. The more diluted blood samples the better purity of the mononuclear cells. Add 35 ml of diluted blood to a 50 ml tube (Fig. 15.1a). Place 10 ml of a density gradient medium with ρ = 1.077 g/ml (e.g. Ficoll-Paque PLUS) at the bottom of the tube with a 15 G hypodermic needle (or similar) (Fig. 15.1b) before centrifugation at 400×g for 25 min at 20 °C in a swinging bucket rotor without brake. Using a brake during centrifugation will disturb the separation of the upper and lower fractions. Aspirate most of the upper layer leaving the mononuclear cells at the interphase (Fig. 15.1c). Carefully transfer the mononuclear cells to a new 50 ml tube, fill the tube with PBS, mix and centrifuge at 300×g for 10 min at 20 °C (Fig. 15.1d). Remove the supernatant completely and resuspend the cells in a small volume of PBS before diluting the cell suspension with 50 ml PBS and centrifuge at 200×g for 15 min at 20 °C. This step will remove platelets, and it should be repeated at least once. When most of the platelets are removed, resuspend the cells in complete RPMI 1640 with 10 % heat inactivated fetal calf serum (FCS), and count them. Cells can be used immediately or preserved and stored in liquid nitrogen.
5.1 Study of Proliferative/Cytotoxic Activity
Dilute the test compounds in RPMI 1640 with additions as described above. Seed the PBMCs at a density of 1 × 105 cells/well in a 96 well plate. Incubate the PBMCs with serial dilutions of the test compound in triplicates at 37 °C in an atmosphere of 5 % CO2 and 100 % humidity. Measurement of effects on proliferative/cytotoxic responses must be done in the presence and absence of the mitogenic stimuli. An array of methods and kit-based assay systems are available for this purpose, for example incorporation of 3H-thymidine or measurement of mitochondrial reductase with tetrazolium salts (MTT). The optimal concentrations of the polyclonal activators can be established by cultivating PBMCs in the presence of twofold dilutions of the activators. Effect of the test compound on proliferation/cytotoxicity should be studied in combination with both optimal and suboptimal concentrations of the mitogenic stimuli. The vehicle control (solvent of the test compound stock) should be included too. As background control, RPMI 1640 with the test compound and activators can be used. As negative control, PBMCs in RPMI 1640 without both test compounds and activators should be used.
5.2 Study of Inflammatory Responses
Upon stimulation with polyclonal activators PBMCs will produce cytokines and up-regulate activation markers. Characterization of the cytokine profile and changes in activation marker expression, especially on T cells, may provide information whether the response is in the direction of Th1, Th2, Th17 or regulatory T cells. Investigations of the cytokine profile is most easily carried out by ELISA analysis of secreted cytokines in the culture supernatant. Alternatively, the number of cells producing the different cytokines can be studied after intracellular staining and analysis by flow cytometry. Also, changes in gene expression levels by qPCR can be informative. Test compounds should be diluted in RPMI 1640. Plate the PBMCs at a density of 1–2 × 106 cells/well in a 24 well plate. Incubate the PBMCs with a serial dilution of the test compound in the presence and absence of LPS (100 ng/ml), anti-CD3/CD28 or the above mentioned mitogenic lectins at 37 °C in an atmosphere of 5 % CO2. Cytokines will be detectable in the culture supernatant after only a few hours. However, cytokines will be secreted with different kinetics, it will therefore be necessary to study the cytokine profile at different time points.
6 Assess Viability
In the case of the study of inflammatory responses, the cytotoxicity of the test compound at varying concentrations should be tested for instance by using LDH or MTT.
7 Experimental Read Out
PBMCs have been widely employed to assess several aspects of immune regulation. The physiological relevance of in vitro studies with PBMCs is debatable, but several reports have shown that these studies can be predictive for the in vivo situation. de Kivit et al. (2012) reported that increased galectin-9 expression in intestinal epithelial cells and serum induced by dietary symbiotics in mice correlated with reduced acute allergic skin reactions and mast cell degranulation. Parallel studies with stimulation of PBMCs with recombinant galectin-9, showed induction of development of Th1 and Treg cells. CD3/CD28-activated PBMCs were incubated with either medium or increasing concentrations of recombinant galectin-9 for 24 h. The effect on T cell activation was assessed by analysis of surface markers by flow cytometry. Th1 cells were identified as CD4+, CD69+ and CXCR3+ and Treg cells as CD4+, CD25+ and Foxp3+. Further characterization of the cytokine profile was used to support the results. Increased secretion of IFNγ and Il-10 suggest increased levels of Th1 and Treg cells, whilst a decrease in IL-17 supports suppression of Th17 cells.
Cytokine secretion is one of the most used outcomes for evaluating influence on immune responses and has been studied with a variety of different types of compounds. For example, Schroecksnadel et al. reported immune modulatory effects of a vitamin K antagonist (Schroecksnadel et al. 2013). PBMCs were pre-incubated with the vitamin K antagonist for 30 min before the cells were stimulated with PHA for 48 h. The cytokines secreted into the supernatants were analyzed by ELISA. Also, in a recent report cytokine secretion and activation of mitogen-activated protein kinases (MAPK) in response to polysaccharides isolated from Alchornea cordifolia (Kouakou et al. 2013) was studied. Evaluation of the effects of polysaccharides on the activation status of an array of different MAPKs was done using a phospho-MAPK kit. PBMCs were incubated with the test compound for 60 min before lysis of the cells. An array of methods and kit based assays are available for evaluation of the activation status of MAPK and other intracellular protein kinases.
Effect of polyphenolic extracts from Carpobrotus rossii on cytokine release from PBMCs has been reported (Geraghty et al. 2011). The cytokine release induced by the polyphenolic extract was investigated in the presence and absence of stimulation with PHA and LPS. In this study, a multiplex cytokine profiling kit (Luminex®) was used to determine cytokine concentrations in culture supernatants.
Several reports have used PBMCs to study the immunomodulatory effects of probiotic bacteria. Lactobacillus plantarum genes involved in immune modulation were identified in a study with PBMCs (van Hemert et al. 2010). A total of 42 L. plantarum strains were evaluated for their capacity to stimulate cytokine production in PBMCs. Comparison of strain-specific cytokine responses by PBMCs resulted in identification of six candidate genetic loci with immune modulatory capacities. Ashraf et al. have recently reported effects of cell-surface extracts and metabolites from a probiotic organism on both cytokine production and induction of CD25 expression on PBMCs (Ashraf et al. 2014).
8 Advantages, Disadvantages and Limitations of the System
The advantage of PBMCs is that it is an easy accessible source of human immune cells, as the cells are isolated form full blood or buffy coats.
Disadvantages and limitations of this model system is the phenotypic differences between peripheral mononuclear cells and immune cells of the intestinal mucosa.
9 Conclusions
Although PBMCs is an easy accessible source of human immune cells, there are two major points that are important to keep in mind. (1) These cells are blood mononuclear cells that will differ from immune cells found i.e. in intestinal tissues. (2) When using PBMC in in vitro experiments the cells will lack the environmental stimuli they would have been exposed to under normal in vivo conditions. Both of these points are of great importance for how immune cells responds to different stimuli and should be taken in to account when interpreting the results.
References
Akdis M, Palomares O, van de Veen W et al (2012) TH17 and TH22 cells: a confusion of antimicrobial response with tissue inflammation versus protection. J Allergy Clin Immunol 129:1438–1449, quiz 1450–1431
Ashraf MT, Khan RH (2003) Mitogenic lectins. Med Sci Monit 9:RA265–RA269
Ashraf R, Vasiljevic T, Smith SC et al (2014) Effect of cell-surface components and metabolites of lactic acid bacteria and probiotic organisms on cytokine production and induction of CD25 expression in human peripheral mononuclear cells. J Dairy Sci 97:2542–2558
Crotty S (2011) Follicular helper CD4 T cells (TFH). Annu Rev Immunol 29:621–663
de Kivit S, Saeland E, Kraneveld AD et al (2012) Galectin-9 induced by dietary synbiotics is involved in suppression of allergic symptoms in mice and humans. Allergy 67:343–352
Geraghty DP, Ahuja KD, Pittaway J et al (2011) In vitro antioxidant, antiplatelet and anti-inflammatory activity of Carpobrotus rossii (pigface) extract. J Ethnopharmacol 134:97–103
Hirahara K, Poholek A, Vahedi G et al (2013) Mechanisms underlying helper T-cell plasticity: implications for immune-mediated disease. J Allergy Clin Immunol 131:1276–1287
Kouakou K, Schepetkin IA, Yapi A et al (2013) Immunomodulatory activity of polysaccharides isolated from Alchornea cordifolia. J Ethnopharmacol 146:232–242
Sakaguchi S, Yamaguchi T, Nomura T et al (2008) Regulatory T cells and immune tolerance. Cell 133:775–787
Schroecksnadel S, Gostner J, Jenny M et al (2013) Immunomodulatory effects in vitro of vitamin K antagonist acenocoumarol. Thromb Res 131:e264–e269
Shale M, Schiering C, Powrie F (2013) CD4(+) T-cell subsets in intestinal inflammation. Immunol Rev 252:164–182
Swiatczak B, Rescigno M (2012) How the interplay between antigen presenting cells and microbiota tunes host immune responses in the gut. Semin Immunol 24:43–49
Tan C, Gery I (2012) The unique features of Th9 cells and their products. Crit Rev Immunol 32:1–10
van Hemert S, Meijerink M, Molenaar D et al (2010) Identification of Lactobacillus plantarum genes modulating the cytokine response of human peripheral blood mononuclear cells. BMC Microbiol 10:293
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Copyright information
© 2015 The Author(s)
About this chapter
Cite this chapter
Kleiveland, C.R. (2015). Peripheral Blood Mononuclear Cells. In: Verhoeckx, K., et al. The Impact of Food Bioactives on Health. Springer, Cham. https://doi.org/10.1007/978-3-319-16104-4_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-16104-4_15
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-15791-7
Online ISBN: 978-3-319-16104-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)