Abstract
The objective was to evaluate the qualification of the new telemetric intracranial pressure (ICP) measurement (t-ICP) device Raumedic® NEUROVENT P-Tel and S-Tel. The proof of concept was examined in a pilot animal study measuring intraperitoneal pressure with a telemetric and a conventional ICP measurement probe at five rates for 1 h each. Moderate external pressure load allowed measuring values between 0 and 40 mmHg. To estimate long-term performance 18 t-ICP devices were implanted subdurally or intraparenchymally into minipigs. Reference measurements were performed regularly using conventional ICP probes. From the short-term as well as from the long-term perspective t-ICP proved to have excellent dynamic ICP signal components perception (e.g. pulse amplitude). Some zero drift of static ICP was found, ranging between 5 and 8 mmHg. While all telemetric, intraparenchymal probes kept their functionality throughout the follow-up, 33% of the subdurals failed for reasons detailed in another paper. Raumedic’s NEUROVENT® P-Tel/S-Tel proved to provide reliable data over periods of up to 18 months. Minor zero drift can be well tolerated as the dynamic ICP signal is measured with excellent stability. Clinicians should focus more on such ICP dynamic signal information than on static ICP when using the device over longer follow-up periods.
Similar content being viewed by others
Keywords
Introduction
For more than four decades attempts have been made to establish a reliable method for telemetric measurement of intracranial pressure (ICP) [1–6]. Yet, as telemetric ICP monitoring requires the use of absolute pressure sensors – one within the implant, one outside to measure the surrounding pressure as a reference – the technical challenge is enormous. Offset-drift on the order of parts per thousand per year in the antidromic direction can result in clinically relevant misleading ICP values within a short time. Only the Cosman-Sensor (Integra Radionics, Burlington, MA, USA) [1, 7], the epidural “Rotterdam Transducer” [2], and the “Aachen-ICP-TeleSensor” (Telemeasurements, Aachen, Germany) [6] reached limited clinical impact.
Major obstacles to generalized acceptance were the inadequacy of some devices in measuring negative pressures, difficult handling, but most of all the lack of reliability. Hence, the risk involved in trusting in telemetrically measured unreliable values outweighed any potential benefits.
Recently new telemetric ICP sensors (NEUROVENT® P-Tel, NEUROVENT® S-Tel for intraparenchymal and subdural measurement, Raumedic AG, Helmbrechts, Germany) have been introduced, demonstrating obviously favourable in vitro performance with minimal drift over years.
We studied the in vivo reliability in one short- and one long-term animal experiment before the now CE-approved devices were implanted in humans. The experiences from these studies and from the first insertion in a patient are reported.
Materials and Methods
Animal Experiment 1
Design
To study the short-term performance of early prototypes of this new telemetric pressure sensor, we measured the intra-peritoneal pressure in five adult, female Wistar rats (weight: 320–350 g) in parallel telemetrically and with conventional, intraparenchymal ICP sensor probes (NEUROVENT-P (diameter: 5F), Raumedic AG, Helmbrechts, Germany). Intraperitoneal pressure was varied by a custom mechanical apparatus, which allowed to apply a large-scale, uniform moderate compression of the rats’ abdominal region. After catheter insertion the resting pressure of the two devices was measured minimally for 5 min. Thereafter, intraperitoneal pressure was varied with different frequencies to estimate the devices’ dynamic response for at least 60 min. Both pressure signals were monitored and stored with a specifically re-designed Raumedic-Datalogger® (Raumedic Reader TDT1 readP, Raumedic AG, Helmbrechts), allowing parallel signal processing of both signals.
Anesthesia and Operative Procedure
Rats were anesthetized using 0.01% (RS)-(±)-2-(2-Chlorphenyl)-2-(methyl-amino)-cyclohexan-1-on (Ketavet®) (2 mL/kg) and 2% 2-(2,6-Dimethylphenylamino)-5,6-dihydro-4H-thiazin (Rompun®) (0.2 mL/kg), intraperitoneally providing sufficient narcosis for about 90 min without the need for artificial ventilation. The electronic part of the telemetric devices was inserted subcutaneously on the flank. Via a median micro-laparotomy the subcutaneously tunneled telemetric catheter and a conventional NEUROVENT-P catheter were inserted intraperitoneally. The peritoneum was firmly closed, applying purse-string sutures. Meticulous subcutaneous stitches and, for superficial closure, 2Octyl-Cyanoacrylat skin adhesive (Dermabond®, ETHICON Products, Norderstedt, Germany) were used.
Animal Experiment 2
Design
To study the long-term performance of the final pre-release versions of the NEUROVENT® P-Tel, NEUROVENT® S-Tel telemetry sensors for at least 1 year, the devices were implanted in nine adult, male Ellegaard (Denmark) minipigs. In each animal one subdural and one parenchymal probe were inserted. As reference a conventional Neurovent-P catheter was also implanted. The correct positioning of the probes was verified by X-ray in different plains, which furthermore served to revise for hydrostatic pressure differences. For data acquisition and storage the Raumedic Reader TDT1 readP was used. In each animal reevaluation occurred regularly after 3 ± 1 and 6 ± 1 months respectively inserting a new reference probe (Neurovent-P) via a new borehole. Again X-ray assessed the probes’ positioning. After implantation (initially and during each control) resting pressure was measured in parallel to the reference for each telemetric probe for at least 10 min. Thereafter, changed positioning (head-up, head-down) provoked ICP alterations. Incline (between +20° and −20°) was measured and recorded electronically by a new designed apparatus whose precision has been verified previously. The same maneuvers were performed separately for each telemetric probe while the reference ICP was measured in parallel. One group (five animals) were euthanized after 9 and 12 months to study the histomorphological alterations of all the surrounding tissues while four minipigs are still alive to evaluate the devices’ in vivo performance for at least 2 years.
Anesthesia and Operative Procedure
Anesthesia was induced with Ketavet® (10%/1 mg/10 kg i.m.) and 2,6-Diisopropylphenol (Propofol®; dosage depending on individual reactions). Given adequate preconditioning, each animal was intubated, artificially ventilated (30% O2, nitrous oxide at 2.5 vol.%) and anesthesia sustained with i.v. N-(1-Phenethyl-4-piperidyl)propionanilide (Fentanyl®; 45–90 μg/kg/h) and vaporized (RS)-Difluormethoxy-1-chlor-2,2,2-trifluorethan (Isofluoran®) at 1.5–2.5 vol.%. Complete monitoring encompassed continuous control of rectal temperature, capillary oxygen saturation (S (O2)), and ECG to maintain physiological values. For hydration left-ear vein i.v. line was used to administer Ringer-Lactate and 0.9% NaCl solutions. For perioperative antibiotic prophylaxis we used amoxicillin i.v. (weight adapted) 30 min before the skin incision, which was continued for a further 3 days. (RS)-2-(6-Chlor-9 H-carbazol-2-yl)propan-acid (Carprofen®) served as post-interventional pain killer.
After skin disinfection with propan-2-ol, 1.0 g povidone-iodine (Braunoderm®) and sterile draping a semicircular skin incision on the fronto-parietal region was made. High-speed drills 5 mm in diameter were used for borehole trephination. The telemetric devices were inserted into the coronal suture level about 1.5 cm paramedian bilaterally, while the borehole for the reference probe was located more frontally. The opening of the tabula interna was minimized, allowing just catheters’ scraping through to avoid cerebrospinal fluid (CSF) loss and preventing pathways for infections. We aimed to place all catheter tips at the same hydrostatic pressure level in neutral prone positioned animals. If necessary, according to X-ray control, the positions were corrected. Typically, the catheter tip ended 1.5 cm below the tabula interna. For the regular invasive controls only a minimal skin incision frontally to the telemetric devices was made to insert the reference sensor.
After finalizing the whole measurement protocol during the initial and all control examinations the reference probe was removed and the dural leak sealed with wax or alpha-gelatin haemostatic sponges. Multilayered subcutaneous stitches and for superficial skin closure Dermabond® were used for wound closure. In addition to its better tolerance the importance of Dermabond® is its bactericide effect, which was demonstrated recently [8].
As implants’ biotolerance was another scope of this study, animals were arbitrarily assigned to a short (3–12 months) or long-term (>12 months) follow-up. In the short-term follow-up group, one, two and one animal were finalized after 3, 6 and 12 months respectively, after undergoing one to three control ICP measurements. To reduce the overall burden of long-term survivors, follow-up examinations were restricted to 3 per year with arbitrary assignment whether the 3, 6 or 9 months’ follow-up was omitted.
All animal experiments were approved by independent animal welfare committees and monitored by the institutional animal welfare delegate. Veterinarians supervised all procedures and performed anesthesia and monitoring-related activities in the minipigs.
Technology
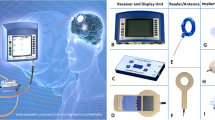
The telemetric implant has a ceramic housing measuring 3 cm in diameter and 4 mm in height, which contains all electronics and the antenna for data and energy transfer (Fig. 1). Technology based on piezoresistive strain gauge silicon-based absolute-pressure sensors with a built-in Wheatstone bridge. ICP changes result in minimal deforming of this chip and the according output voltage changes. In parallel, the tip sensor measures local temperature. Both signals become digitized by the ASIC within the ceramic housing. During the manufacturers’ individual calibration of each device 7 chip-specific parameters are generated and permanently saved on the ASIC to ensure maximal reliability. Without external energy input the implant remains inactive. The Raumedic Reader TDT1 readP identifies using RFID (radio-frequency identification) technology each individual implant and its specific calibration data. The externally applied antenna of the reader initially supplies the energy required to initiate the implants’ measurement, which is received by the antenna too. This process of energy transfer and data transmission allows sampling at 5 Hz and a resolution of 1 mmHg. The working range encompasses −20 to +400 mmHg. Once the data package of absolute ICP and brain temperature has been transferred, the Datalogger® computes the relative ICP by parallel measurement of the surrounding (atmospheric) pressure with a built-in second absolute, temperature-corrected pressure sensor. This computed relative ICP is displayed on the Datalogger’s screen and stored on a built-in SSDS. A built-in power supply and the low weight of the Raumedic Datalogger Reader TDT1 readP allows ambulatory on-demand ICP measurement by patients by themselves after briefing.
Schematic drawing of the telemetric NEUROVENT® P-Tel device. The ASIC for data processing and exchange and the antenna for energy and data transfer are located in a ceramic housing. A piezoresistive strain gauge with silicon-based absolute-pressure sensors and built-in Wheatstone bridge measures the ICP on the tip of the 5F (diameter) catheter
Data Management and Statistics
The software packages: Microsoft-Excel 2003 (Redmond, USA), PASW Statistics 18 (SPSS Inc, an IBM Company, Chicago, USA), Raumedic-Datalog® (RECO-Medizintechnik, Pirna, Germany), and WinSTAT® for Excel Version 2009.1 (R. Fitch Software, Bad Krozingen, Germany) were used for data management, analysis, and computation. Significance was assumed at p < 0.05.
Results
Animal Experiment 1
Intraperitoneal pressures between 0 and 40 mmHg could be measured with both the telemetric and the conventional probe. Telemetrically and conventionally measured pressure correlated in an excellent manner (p < 0.000), the maximal difference between the two was 1.8 ± 0.8 mmHg on average in one animal. In the others the mean difference was even smaller. Overall, during 310 min monitoring in five animals the mean pressure difference between control and telemetric probe measured was 1.1 ± 0.7 mmHg. The dynamic characteristics of the pressure signal as measured with both devices were nearly identical. Despite the 5-Hz sampling rate of the telemetric probe, the pulse amplitude could be measured precisely, with an overall pressure difference of 0.4 ± 0.3 mmHg on average and pulse amplitudes ranging between 1 and 6 mmHg and artificially induced (by short-term manual pressure) short-term ICP variations up to 15 mmHg during 1 s (p < 0.000).
Animal Experiment 2
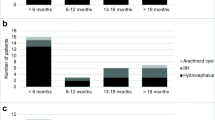
Overall 12 subdural and 8 intraparenchymal telemetric devices were implanted in 9 minipigs (7 had both subdural and intraparenchymal probes; 2 had bilaterally subdural probes). During the observational period a total of 25 control measurements, 750 monitored minutes in total, were performed telemetrically and conventionally. Between 3 and 6 (group 1), 7 and 12 (group 2) and 18 (group 3) months after implantation 8, 11 and 6 controls were performed. While all implanted parenchymal probes are still functioning or reached the regular endpoint, 4 of the 12 implanted subdural probes failed (Fig. 2). Detailed descriptive statistics of the ICP differences measured between the reference and the telemetric probe are provided in Table 1. Excluding outliers at the level of one standard deviation (SD) or more provides more realistic data. Under these conditions pressure differences measured between the telemetric and the reference probe were 5.5 ± 4.0 mmHg (group 1), 4.5 ± 4.7 mmHg (group 2), and 8.3 ± 3.7 mmHg (group 3). Neither ANOVA (p = 0.853) nor the Kruskal–Wallis test (p = 0.717) revealed a difference between the groups. As the groups stand for different follow-up periods when controls were performed, this result must be interpreted as a sign of measurement stability without increasing zero drift with time (Fig. 3). The most impressive finding was the reliability of the signals’ dynamic content measurement. Even probes with significant zero shift (outliers) provided comparable ICP signal dynamics, including pulse amplitude and ICP alterations due to positioning. The mean coefficients of determination (R 2), were calculated for groups 1 (first control (3–6 months after insertion)), 2 (second control (12 months after insertion)), and 3 (third control (18 months after insertion)) to be 0.899 ± 0.119, 0.826 ± 0.225, and 0.917 ± 0.113 respectively, indicating excellent signals’ dynamic ICP components perception during the whole follow-up period (ANOVA: p = 0.520; Kruskal-Wallis: p = 0.342; Fig. 3). Hence, even if the static ICP value is somewhat affected by zero drift, the excellent stability of the dynamic ICP signal content provides sufficient and reliable data over time to recognize alterations in cranio-spinal CSF dynamics.
Example of 12-month control of telemetrically (intraparenchymal) and conventionally measured ICP during 13 min. Effects of positioning (neutral, head-up, neutral, two steps head-down) clearly provoke corresponding ICP changes. The average pressure difference between control and telemetric probe was 0.5 mmHg. The perfect correlation of control and telemetric ICP measurement is also represented by the according scatter plot of both measured values (control on the y-axis, telemetric on the x-axis) in the smaller graphic
Initial Clinical Experiences
As the NEUROVENT P-Tel has been CE-approved recently for clinical usage over a maximal period of 29 days, we were able to use such a device to clarify recurrent headache in an adolescent girl shunted for hydrocephalus. A multitude of clinical interventions (e.g. conventional ICP measurement, infusion tests, probationary shunt revision) have failed before, but meticulous clinical examinations could not find any explanation for her headache other than intermittent shunt failure. The telemetric device was inserted left frontally and the patient was instructed how to measure ICP telemetrically at home when she suffers headache, but she was also advised to measure regularly three times per day if she had no complaints. Thus, an overall data record of about 4 h (2.5 h without complaints; 1.5 h with headache) could be gathered over 28 days. Neither the dynamic nor the static ICP differed between episodes with and without complaints, proving that headaches were independent of ICP. All the data measured were reliable and the device proved its qualification for ambulatory ICP measurement by patients themselves. According to the specification the device was explanted on day 29. No side effects occurred throughout the whole treatment. We found this methodology the most useful measure to exclude intermittent shunt failure.
Discussion
During the past four decades at least 30 prototypes for telemetric ICP measurement have been proposed [1–6, 9, 10], but only minority have reached limited clinical impact. In some of them, their failure can be well understood from a retrospective perspective. Foreign body reactions with calcifications must damp any transdural signal transfer in long-term epidural implants for telemetric ICP measurement. The integration of such devices into shunts and accordingly the CSF-coupled measurement was at risk of dangerous ICP underestimation at proximal catheter obstruction. The difficulty is that ICP pulsatility can be visible, yet because soft silicone tubes are compressible and some pulsation can be transferred to the decoupled water column, mimicking reliable ICP measurements. Accordingly, reliable telemetric ICP measurement needs intradural, tip-sensor-based data acquisition as realized with the Raumedic NEUROVENT® P-Tel and S-Tel probes. As our interim data analysis demonstrates, intraparenchymal probes in particular provide reliable data for up to 18 months. The median offset drift (range: 5–8 mmHg) remained quite stable during the follow-up of a maximum of 18 months. However, much more importantly: even devices with distinct zero shift of the static pressure signal maintained reliable dynamic signal acquisition. Even ICP pulse amplitude was correctly measured. This is of utmost importance for long-term monitoring of shunted patients. Knowing the dynamic signal characteristics resulting from physiological ICP variations (heartbeat, breathing) and positioning with a well-functioning shunt, Shunt failures must result in alterations in dynamic signal characteristics, as consequence of altered CSF-hydrodynamics.
The fundamental idea of developing subdural telemetric probes was to avoid any brain tissue violation as occurs with intraparenchymal probes. In our examination >30% of the subdural probes failed or provided unreliable data 6–10 months after implantation. Potential explanations have been detailed previously [11]. Hence, as not all subdural probes failed and have been examined, final statements on its qualification are pending. Probably, the comparatively small cranium of minipigs effectuates higher tensile stress on subdural probes, which does not occur in humans as they have a lower radius of curvature and larger skulls. Yet, in the clinical setting, we actually prefer intraparenchymal probes as a result of our findings. Almost negligible histomorphological alterations around 12 months in intraparenchymal probes implanted into minipigs support such a policy until all subdural probes have been examined.
Conclusion
The new Raumedic NEUROVENT® P-Tel and S-Tel telemetric ICP measurement device has been proven to provide reliable data for up to 1.5 years. Even if some zero shift occurs over time the dynamic signal content remains unaffected and of outstanding reliability.
References
Cosman ER, Zervas NT, Chapman PH, Cosman BJ, Arnold MA (1979) A telemetric pressure sensor for ventricular shunt systems. Surg Neurol 11:287–294
de Jong DA, Berfelo MW, de Lange SA, Maas AI (1979) Epidural pressure monitoring with the so-called Rotterdam transducer. Further in vivo results. Acta Neurochir (Wien) 45:301–309
Heppner F, Lanner G, Rodler H (1976) Telemetry of intracranial pressure. Acta Neurochir (Wien) 33:37–43
Zervas NT, Cosman ER, Cosman BJ (1977) A pressure-balanced radio-telemetry system for the measurement of intracranial pressure. A preliminary design report. J Neurosurg 47:899–911
Rylander HG, Taylor HL, Wissinger JP, Story JL (1976) Chronic measurement of epidural pressure with an induction-powered oscillator transducer. J Neurosurg 44:465–478
Richard KE, Block FR, Weiser RR (1999) First clinical results with a telemetric shunt-integrated ICP-sensor. Neurol Res 21:117–120
Wilson MH, Milledge JMD (2008) Direct measurement of intracranial pressure at high altitude and correlation of ventricular size with acute mountain sickness: Brian Cummins’ results from the 1985 Kishtwar expedition. Neurosurgery 63:970–975
Eymann R, Kiefer M (2010) Glue instead of stitches: a minor change of the operative technique with a serious impact on the shunt infection rate. Acta Neurochir Suppl 106:87–89
Miyake H, Ohta T, Kajimoto Y, Matsukawa M (1997) A new ventriculoperitoneal shunt with a telemetric intracranial pressure sensor: clinical experience in 94 patients with hydrocephalus. Neurosurgery 40:931–935
Osaka K, Ohta T (1980) Limits of various methods for evaluation of shunt function and development of new intracranial pressure meter incorporated in the shunt system. No Shinkei Geka 8:811–817
Schmitt M, Eymann R, Antes S, Kiefer M (2011) Subdural or intraparenchymal placement of long-term telemetric ICP measurement devices. Acta Neurochir Suppl: Hydrocephalus, 113. Aygok, Gunes A, Rekate, Harold L. (Eds.); 2012, XII, ISBN 978-3-7091-0922-9
Financial Disclosure
This study has been performed with support of the German Ministry for Research and Education, which was granted to a consortium of the Saarland University (M. Kiefer, S. Antes, M. Schmitt, R. Eymann), the RWTH Aachen (Steffen Leonhardt), and Raumedic® AG (Helmbrechts, Germany) (BMBF-Grant ID-No.: 16SV3745). These authors have received no further financial benefits from this research work. B. Orakcioglu received some financial support from Raumedic AG for performing the study. No aspects of these studies (design, data acquisition and analysis or content of this manuscript) were influenced by any of the sponsors or the industrial partner of the consortium.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag/Wien
About this chapter
Cite this chapter
Kiefer, M. et al. (2012). Telemetric ICP Measurement with the First CE-Approved Device: Data from Animal Experiments and Initial Clinical Experiences. In: Schuhmann, M., Czosnyka, M. (eds) Intracranial Pressure and Brain Monitoring XIV. Acta Neurochirurgica Supplementum, vol 114. Springer, Vienna. https://doi.org/10.1007/978-3-7091-0956-4_20
Download citation
DOI: https://doi.org/10.1007/978-3-7091-0956-4_20
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-0955-7
Online ISBN: 978-3-7091-0956-4
eBook Packages: MedicineMedicine (R0)