Abstract
Acute myelogenous leukemia (AML) is derived from self-renewing leukemic stem cells (LSCs). We found that T-cell immunoglobulin mucin-3 (TIM-3) is expressed on LSCs in most types of primary AML, except for acute promyelocytic leukemia (M3 by the FAB classification). TIM-3 is not expressed in normal hematopoietic stem cells (HSCs). In a xenogeneic transplantation system, we showed that targeting of TIM-3 by an anti-TIM-3 cytotoxic antibody is sufficient to eradicate human AML LSCs without affecting normal human hematopoiesis. These data strongly suggest that TIM-3 is a promising therapeutic target to cure AML patients.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
- Acute myelogenous leukemia
- Leukemic stem cell
- Cancer stem cell
- TIM-3
- Hematopoietic stem cell
- Xenotransplantation
Introduction
In normal hematopoiesis, human hematopoietic stem cells (HSCs) reside within the CD34+CD38− cell fraction of bone marrow cells. They self-renew and differentiate into mature cells to maintain normal hematopoiesis. Similarly, in acute myelogenous leukemia (AML), leukemic blast cells are derived from a small population called leukemic stem cells (LSCs) or leukemia-initiating cells, which also resides within the CD34+CD38− cell fraction [1, 2]. LSCs self-renew and give rise to clonogenic leukemic cells, whereas non-LSCs lack the potential to self-renew or maintain leukemia [1, 3, 4] indicating that AML is hierarchically organized initiating from LSCs.
Conventional chemotherapy currently achieves complete remission in ~90 % of AML cases [5, 6]. However, a considerable proportion of AML patients (~60 %) eventually relapse after intensive chemotherapies. The recurrence of AML in these patients may be caused by regrowth of surviving LSCs. To selectively kill AML LSCs while sparing normal HSCs, one of the most practical approaches is to target AML LSC-specific surface molecules or molecules required for LSC function. To achieve such specificity, the target molecule should be expressed on LSCs at a high level but not on normal HSCs [7]. The molecule can be expressed in mature blood cells or progenitors, because these cells can anyway be replenished by normal HSCs.
Search for Surface Antigens Specific to AML LSCs
A number of candidate surface molecules for eradicating AML LSCs have been reported mainly by utilizing cDNA microarray analysis of purified LSCs. Figure 1 shows the results of transcriptome profiling of purified LSCs from AML patients and normal adult HSCs [8]. The molecules strongly expressed in AML LSCs, including CLL-1 [9], CSF1R [10], CD96 [11], and CD99 [12], are specifically expressed in LSCs. CLL-1 is a transmembrane glycoprotein [13]. The proportion of CLL-1-expressing CD34+CD38− AML cells, however, is highly diversified in cases [9]. CD96 is a member of the Ig gene superfamily. CD96 is expressed on activated T cells [14]. The expression level of CD96 protein is also high enough to clearly distinguish AML LSCs from normal HSCs. T-cell immunoglobulin mucin-3 (TIM-3) is expressed in LSCs of most AML types (except for M3) at high levels, but is not expressed in normal HSCs [8]. The expression level of CD25 [15], CD32 [15], CD44 [16], and CD47 [17] in LSCs was only two- to threefold higher at the mRNA level as compared with normal HSCs, and in some AML cases, LSCs did not express these molecules. CD33 and CD123 [18] proteins are expressed at a high level in normal HSCs and myeloid progenitors, including CMPs and GMPs [19], suggesting that targeting these molecules should harm normal hematopoiesis.
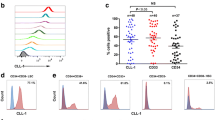
TIM-3 expression in normal HSCs and AML LSCs. (a) Results of gene expression analysis comparing CD34+CD38− normal HSCs and AML LSCs. Surface molecules highly expressed in LSCs are shown. (b) FACS analysis of TIM-3 protein expression in normal HSCs and AML LSCs. Both CD34+CD38−CD90− LSCs and CD34+CD38+AML cells express TIM-3, whereas CD34+CD38−CD90− HSCs completely lack TIM-3 expression. TIM-3 expression originates within the CD34+CD38+ progenitor fraction in normal human hematopoiesis. A representative FACS analysis is shown here
It might also be important to understand the function of these therapeutic target molecules in the development of AML. A previous study has shown that anti-CD44 monoclonal antibodies reduced the leukemic burden and blocked secondary engraftment in a NOD-SCID model [16]. This effect on LSCs was mediated in part by the disruption of LSC-niche interactions [16]. Anti-CD47 antibodies can block LSC reconstitution in a NOD-SCID model [17], and this might be due to the activation of phagocytosis by macrophages through inhibition of interaction of CD47 with SIRPA [20].
Recently, we have reported that TIM-3 is expressed on the cell surface of LSCs in most AML types [8, 21]. TIM-3 is not expressed in normal human HSCs [8] (Fig. 1). Furthermore, a recent study has succeeded in prospectively isolating LSCs from residual HSCs within the CD34+CD38− fraction in de novo AML patients by using TIM-3 as a positive LSC marker [12]. Here, we summarize recent progress in studies of TIM-3 and discuss the potential usefulness of TIM-3 for eradicating AML LSCs. TIM-3 has several advantages over other candidate markers. First, TIM-3 protein is not detectable in normal HSCs or in other myelo-erythroid or lymphoid progenitors, although TIM-3 is upregulated in monocyte-lineage committed progenitors. Second, TIM-3 marks all functional LSCs that can reconstitute human AML in immunodeficient mice in the majority of M0, M1, M2, and M4 AML cases, and its expression level is sufficient to eradicate LSCs by antibody-based treatment.
TIM-3 Expression and Functions in Normal Hematopoiesis
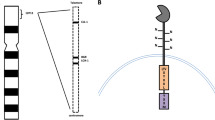
TIM-3 was originally identified as a surface molecule expressed in interferon (IFN)- γ-producing CD4+ Th1 cells and CD8+ T cytotoxic type 1 (Tc1) cells [22] in murine hematopoiesis. TIM-3, a type 1 cell-surface glycoprotein, has a structure that includes an N-terminal immunoglobulin variable domain followed by a mucin domain, a transmembrane domain, and a cytoplasmic tail (Fig. 2). In steady-state human hematopoiesis, TIM-3 is expressed in monocytes and in a fraction of NK cells, but not in granulocytes, B cells, or T cells [8]. However, TIM-3 is upregulated in T cells in response to immune reactions. TIM-3 plays an important role in regulation of Th1-dependent immune responses and immune tolerance [22–24]. Galectin-9, an S-type lectin, has been reported as a TIM-3 ligand in lymphocytes. Galectin-9 has two distinct carbohydrate recognition domains and binds to carbohydrate chains on the IgV domain of TIM-3. TIM-3 has highly conserved six tyrosine residues and a Src homology 2 (SH2) binding motif in its cytoplasmic tail, and stimulation of TIM-3 by galectin-9 results in increased phosphorylation of tyrosine residues in T cells [25]. Engagement of TIM-3 by galectin-9 induces apoptosis of Th1 cells and inhibits their IFN-γ production [26]. These data collectively suggest that TIM-3 is a negative regulator of Th1- and Tc1-driven immune responses.
Structure of TIM-3 molecule and its ligands. TIM-3 is a type 1 cell-surface glycoprotein and has a structure that includes an N-terminal immunoglobulin variable domain followed by a mucin domain, a transmembrane domain, and a cytoplasmic tail with highly conserved six tyrosine residues and a SH2 binding motif. Galectin-9, HMGB1, and PS have been identified as ligands of TIM-3
TIM-3 is also known as a marker of “exhausted” CD8+ T cells. Exhausted T cells show impaired proliferation and effector function under antigen stimulation. One of the major markers for exhausted T cells is the inhibitory molecule programmed cell death 1 (PD-1), and T cell function is partially restored by blocking the interaction between PD-1 and PD-1 ligand in mice [27]. TIM-3 is also expressed on exhausted CD8+ T cells in patients with chronic viral infections, including human immunodeficiency virus (HIV) [28], hepatitis B virus [29], and hepatitis C virus (HCV) [30]. Blockade of both TIM-3 and PD-1 ligation can significantly restore T cell proliferation and effector potential, suggesting that both TIM-3 and PD-1 pathways play a major role in CD8+ T cell exhaustion [31].
TIM-3 can also modulate the immune reaction pathway to regulate innate immunity. NK cells and some myeloid cells, including monocytes/macrophages, dendritic cells, and mast cells, express TIM-3 in both human and mouse hematopoiesis. In NK cells, TIM-3 is induced on their surface on activation [32, 33], but the function of TIM-3 in NK cells remains controversial. It has been reported that TIM-3 is a human NK cell co-receptor to enhance IFN-γ production [32], but another report showed that NK cell-mediated cytotoxicity was reduced by cross-linking of TIM-3 [33].
In terms of the myeloid lineage, TIM-3 is expressed in monocytes/macrophages, dendritic cells (DCs), and mast cells [34–37]. In human bone marrow, CD34+CD38−CD90+ normal HSCs and the majority of CD34+CD38+ progenitor cells do not express TIM-3. Within the CD34+CD38+ progenitor fraction, human myeloid progenitors can be divided into three subpopulations, such as common myeloid progenitors (CMPs), granulocyte/macrophage progenitors (GMPs), and megakaryocyte/erythrocyte progenitors (MEPs) [38]. TIM-3 is expressed only in a fraction GMPs, but not in CMPs and MEPs. Purified TIM-3+ GMPs give rise mainly to colony-forming unit-macrophage (CFU-M), whereas TIM-3− GMPs can generate various types of myeloid colonies, suggesting that upregulation of TIM-3 occurs in concert with monocyte lineage commitment at the GMP stage in humans [8].
In mature monocytes or dendritic cells, TIM-3 signaling synergizes with that of Toll-like receptors to promote secretion of tumor necrosis factor-α (TNF-α) inflammatory responses [34]. In addition, TIM-3 on macrophages and DCs recognizes phosphatidylserine (PS) in apoptotic cells through its IgV domain. Binding of PS to TIM-3 does not interfere with that of galectin-9 to TIM-3, as the binding sites of these molecules are located on opposite sides of the IgV domain. In TIM-3-expressing DCs, recognition of PS by TIM-3 induced enhancement of phagocytosis of apoptotic cells and cross-presentation of apoptotic cell-associated antigen to CD8+ T cells [35]. TIM-3 expression and functions in hematopoietic cells are summarized in Fig. 3.
TIM-3 Is a Marker of Malignant Stem Cells in Human AML
We have identified TIM-3 as an AML LSC-specific surface molecule. We first compared the gene expression profiles of CD34+CD38− AML cells and normal HSCs by using cDNA microarray analysis (Fig. 1a). As shown in Fig. 1b, TIM-3 protein is not expressed in CD34+CD38−CD90+ normal HSCs, but the vast majority of the CD34+CD38− LSCs and the CD34+CD38+ cells expressed TIM-3 at a high level in patients with most types of AML except for acute promyelocytic leukemia (M3) [8, 21]. Another group has also reported that the expression level of TIM-3 is especially high in immature AML cells with core-binding factor translocations or mutations in CEBPA [21].
It is important to note that the TIM-3+ fraction in AML patients contained all functional LSCs. We separated AML cells into the TIM-3+ and TIM-3− populations and transplanted each population into sublethally irradiated immunodeficient mice, and found that only TIM-3+ AML cells, but not TIM-3− cells, reconstituted human AML in these mice [8]. These data suggest that targeting TIM-3+ cells is sufficient for eradication of LSCs in AML patients.
Targeting AML-LSCs by Monoclonal Anti-TIM-3 Killing Antibodies in a Xenograft Model
To utilize TIM-3 to target AML LSCs, it is critical to establish anti-human TIM-3 antibodies that can kill TIM-3-expressing cells in vivo. To achieve successful antibody-based treatment, antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) activities are critical to eliminate target cells [39]. Additionally, recent studies have suggested that antibody-dependent cellular phagocytosis (ADCP) could play an important role in killing target cells in vitro [40] and in vivo [41].
An anti-TIM-3 monoclonal antibody (IgG2b) was obtained by immunizing Balb/c mice with L929 cells stably expressing human TIM-3 and soluble TIM-3 protein [8]. In this antibody, the variable portions of the VH regions of the cloned hybridoma that recognize TIM-3 were grafted onto IgG2a Fc regions, because the IgG2a subclass is most efficient to induce ADCC activity in mice [42, 43]. The clone called ATIK2a was established, and it was effective in killing TIM-3-expressing cell lines by its CDC and ADCC activities [8].
We then tested the effect of ATIK2a on the growth of AML LSCs or normal HSCs in xenograft models. NOD-SCID mice transplanted with 105 CD34+ cord blood cells were treated with ATIK2a. These mice were reconstituted with normal hematopoiesis with nearly equal percentages of human cell chimerisms, although human mature monocytes were depleted. In contrast, in mice reconstituted with human AML, ATIK2a exerted profound effects on leukemia development. The mice were transplanted with human AML of M0, M1, and M4 types, and after confirmation of AML development in these mice, ATIK2a was injected six times over 2 weeks. Strikingly, human AML cells disappeared in mice treated with ATIK2a but not in those with control IgG treatment. These data strongly suggest that targeting of AML LSCs by utilizing anti-TIM-3 killing antibodies is a practical approach to cure human AML.
TIM-3 Is a Functional Molecule for AML LSC Maintenance
Since TIM-3 has a tyrosine residue and SH2 domain that can activate Src family proteins, we hypothesized that TIM-3 signaling has some function to maintain AML-LSCs. We found that the serum levels of galectin-9, a TIM-3 ligand, were significantly (>10-fold) elevated in AML patients but not in normal individuals on an ELISA assay. Furthermore, TIM-3+ AML cells had abundant galectin-9 protein in their cytoplasm, and they secreted galectin-9 in the sera of mice transplanted with human AML. Mice reconstituted with normal human HSCs or B cell acute lymphoblastic leukemia did not have detectable levels of serum galectin-9. These results collectively suggest that AML cells secreted galectin-9 in an autocrine manner. Furthermore, TIM-3 stimulation by galectin-9 in AML cells in vitro induced significant gene expression changes including NF-κB target genes (unpublished data). Collectively, it is suggested that AML LSCs had growth and survival advantages through an autocrine stimulation loop of the TIM-3/galectin-9 system.
Conclusion
TIM-3 has been shown to play pivotal roles in modulating immune reactions. By transcriptome analysis, we newly identified TIM-3 as a surface molecule specific to AML LSCs but not expressed in normal HSCs. Our in vivo xenogeneic transplantation analysis directly showed that targeting TIM-3 could be an efficient, useful therapeutic approach to eradicate AML LSCs.
References
Lapidot T et al (1994) A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367(6464):645–648
Ishikawa F et al (2007) Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol 25(11):1315–1321
Bonnet D, Dick JE (1997) Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 3(7):730–737
Hope KJ, Jin L, Dick JE (2004) Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol 5(7):738–743
Miyawaki S (2012) Clinical studies of acute myeloid leukemia in the Japan Adult Leukemia Study Group. Int J Hematol 96(2):171–177
Stein EM, Tallman MS (2012) Remission induction in acute myeloid leukemia. Int J Hematol 96(2):164–170
Krause DS, Van Etten RA (2007) Right on target: eradicating leukemic stem cells. Trends Mol Med 13(11):470–481
Kikushige Y et al (2010) TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell 7(6):708–717
van Rhenen A et al (2007) The novel AML stem cell associated antigen CLL-1 aids in discrimination between normal and leukemic stem cells. Blood 110(7):2659–2666
Aikawa Y et al (2010) PU.1-mediated upregulation of CSF1R is crucial for leukemia stem cell potential induced by MOZ-TIF2. Nat Med 16(5):580–585, 1p following 585
Hosen N et al (2007) CD96 is a leukemic stem cell-specific marker in human acute myeloid leukemia. Proc Natl Acad Sci U S A 104(26):11008–11013
Jan M et al (2012) Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Sci Transl Med 4(149):149ra118
Bakker AB et al (2004) C-type lectin-like molecule-1: a novel myeloid cell surface marker associated with acute myeloid leukemia. Cancer Res 64(22):8443–8450
Wang PL et al (1992) Identification and molecular cloning of tactile. A novel human T cell activation antigen that is a member of the Ig gene superfamily. J Immunol 148(8):2600–2608
Saito Y et al (2010) Identification of therapeutic targets for quiescent, chemotherapy-resistant human leukemia stem cells. Sci Transl Med 2(17):17ra9
Jin L et al (2006) Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med 12(10):1167–1174
Majeti R et al (2009) CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 138(2):286–299
Jin L et al (2009) Monoclonal antibody-mediated targeting of CD123, IL-3 receptor alpha chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell 5(1):31–42
Taussig DC et al (2005) Hematopoietic stem cells express multiple myeloid markers: implications for the origin and targeted therapy of acute myeloid leukemia. Blood 106(13):4086–4092
Takenaka K et al (2007) Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol 8(12):1313–1323
Jan M et al (2011) Prospective separation of normal and leukemic stem cells based on differential expression of TIM3, a human acute myeloid leukemia stem cell marker. Proc Natl Acad Sci U S A 108(12):5009–5014
Monney L et al (2002) Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 415(6871):536–541
Sanchez-Fueyo A et al (2003) Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol 4(11):1093–1101
Sabatos CA et al (2003) Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol 4(11):1102–1110
van de Weyer PS et al (2006) A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9. Biochem Biophys Res Commun 351(2):571–576
Zhu C et al (2005) The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol 6(12):1245–1252
Barber DL et al (2006) Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439(7077):682–687
Jones RB et al (2008) Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med 205(12):2763–2779
Wu W et al (2012) Blockade of Tim-3 signaling restores the virus-specific CD8(+) T-cell response in patients with chronic hepatitis B. Eur J Immunol 42(5):1180–1191
Golden-Mason L et al (2009) Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol 83(18):9122–9130
Sakuishi K et al (2010) Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 207(10):2187–2194
Gleason MK et al (2012) Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood 119(13):3064–3072
Ndhlovu LC et al (2012) Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 119(16):3734–3743
Anderson AC et al (2007) Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science 318(5853):1141–1143
Nakayama M et al (2009) Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood 113(16):3821–3830
Nakae S et al (2007) TIM-1 and TIM-3 enhancement of Th2 cytokine production by mast cells. Blood 110(7):2565–2568
Dekruyff RH et al (2010) T cell/transmembrane, Ig, and mucin-3 allelic variants differentially recognize phosphatidylserine and mediate phagocytosis of apoptotic cells. J Immunol 184(4):1918–1930
Manz MG et al (2002) Prospective isolation of human clonogenic common myeloid progenitors. Proc Natl Acad Sci U S A 99(18):11872–11877
Nimmerjahn F, Ravetch JV (2007) Antibodies, Fc receptors and cancer. Curr Opin Immunol 19(2):239–245
Manches O et al (2003) In vitro mechanisms of action of rituximab on primary non-Hodgkin lymphomas. Blood 101(3):949–954
Oflazoglu E et al (2009) Macrophages and Fc-receptor interactions contribute to the antitumour activities of the anti-CD40 antibody SGN-40. Br J Cancer 100(1):113–117
Nimmerjahn F, Ravetch JV (2005) Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science 310(5753):1510–1512
Uchida J et al (2004) The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med 199(12):1659–1669
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Copyright information
© 2015 The Author(s)
About this paper
Cite this paper
Akashi, K. (2015). TIM-3 Is a Novel Therapeutic Target for Eradicating Acute Myelogenous Leukemia Stem Cells. In: Nakao, K., Minato, N., Uemoto, S. (eds) Innovative Medicine. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55651-0_25
Download citation
DOI: https://doi.org/10.1007/978-4-431-55651-0_25
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55650-3
Online ISBN: 978-4-431-55651-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)