Abstract
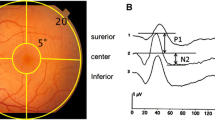
Studies of the focal macular electroretinogram (ERG) have been made with special reference to oscillatory potentials (OPs) by using a fundus monitoring system in humans. Human macular OPs consist of 3 to 4 wavelets (mean peak interval, approximately 6.5 msec). The distribution of OPs in relation to those in a- and b-waves was studied. The amplitudes of a-waves, b-waves, and OPs of the upper macula were significantly larger than those of the lower macula. The distribution of OPs is relatively sparse in the fovea, becoming more dense than the a- and b-waves from the fovea toward the parafovea, and differing even more toward the perifovea. There was no statistical difference of amplitude in a- and b-waves between nasal and temporal macula. The amplitude of OPs in the temporal macula, however, was significantly larger than in the nasal macula. In some macular diseases, such as diabetic maculopathy, cystoid macular edema, or the convalescent stage of central serous chorioretinopathy, macular OPs were selectively reduced, leaving the a- and b-waves intact. Macular OPs can provide a new aspect of macular function and can be a sensitive indicator to assess that function in macular diseases.
Similar content being viewed by others
References
Cobb WA, Morton HB. A new component of the human electroretinogram. J Physiol 1954; 123: 36–37.
Yonemura D. The oscillatory potentials of the electroretinogram. Acta Soc Ophthalmol 1962; 66: 1566–84.
Speros P, Price J. Oscillatory potentials. History, techniques and potential use in the evaluation of disturbances of retinal circulation. Surv Ophthalmol 1981; 25: 237–52.
Brown KT. The electroretinogram: Its components and their origin. Vision Res 1968; 8: 633–77.
Wachtmeister L, Dowling JE. The oscillatory potentials of the mud puppy retina. Invest Ophthalmol 1978; 17: 1176–88.
Yonemura D, Kawasaki K. Electrophysiological study on activities of neuronal and nonneuronal retinal elements in man with reference to its clinical application. Jpn J Ophthalmol 1978; 22: 195–213.
Haynen N, Wachtmeister L, van Norren D. Origin of the oscillatory potentials in the primate retina. Vision Res 1985; 10: 1365–73.
Arden GB, Bankes JLK. Foveal electroretinogram as a clinical test. Br J Ophthalmol 1966; 50: 740.
Biersdorf WR, Diller DA. Local electroretinogram in macular degeneration. Am J Ophthalmol 1969; 68: 296–303.
Jacobson JH, Kawasaki K, Hirose T. The human electroretinogram and occipital potential in response to focal illumination of the retina. Invest Ophthalmol 1969; 8: 545–56.
Nagata M, Honda Y. Studies on the local electric response of the human retina. 1. An instrument for stimulating local retinal areas in various photopic conditions. Acta Soc Ophthalmol Jpn 1970; 74: 388–94.
van Lith GHM, Henkes HE. The relationship between ERG and VER. Ophthalmol Res 1970; 1: 40–7.
Hirose T, Miyake Y, Hara A. Simultaneous recording of electroretinogram and visual evoked response. Focal stimulation under direct observation. Arch Ophthalmol 1977; 95: 1205–8.
Sandberg MA, Effron MH, Berson EL. Foveal cone electroretinograms in dominant retinitis pigmentosa with reduced penetrance. Invest Ophthalmol 1978; 17: 1096–101.
Miyake Y, Yanagida K, Yagasaki K, Kondo S. Subjective scotometry and recording of local electroretinogram and visual evoked response. System with television monitor of the fundus. Jpn J Ophthalmol 1981; 25: 438–48.
Miyake Y, Awaya S. Stimulus deprivation amblyopia. Simultaneous recording of local macular electroretinogram and visual evoked response. Arch Ophthalmol 1984; 102: 998–1003.
Miyake Y. Studies of local macular ERG. Acta Soc Ophthalmol Jpn 1988; 92: 1419–49.
Miyake Y, Shiroyama N, Ota I, Horiguchi M. Oscillatory potentials in electroretinograms of the human macular region. Invest Ophthalmol Vis Sci 1988; 29: 1631–5.
Miyake Y, Shiroyama N, Ota I, Horiguchi M. Local macular electroretinographic responses in idiopathic central serous chorioretinopathy. Am J Ophthalmol 1988; 106: 546–50.
Miyake Y, Shiroyama N, Horiguchi M, Ota I. Asymmetry of focal ERG in human macular region. Invest Ophthalmol Vis Sci, 1989; 30: 1743–49.
Miyake Y, Ichikawa K, Shiose Y, Kawase Y. Hereditary macular dystrophy without visible fundus abnormality. Am J Ophthalmol, 1989; 108: 292–99.
Eason RG, White CT, Oden D. Averaged occipital responses to stimulation of sites in the upper and lower halves of the retina. Percept Psychophys 1967; 2: 423–5.
Lehmann D, Skrandies W. Multichannel evoked potential fields show different properties of human upper and lower hemiretina system. Expl Brain Res 1979; 35: 151–9.
Skrandies W, Richter M, Lehmann D. Checkerboard evoked potentials: Topography and latency for onset, offset, and reversal. Prog Brain Res 1980; 54: 291–5.
Adachi-Usami E, Lehmann D. Monocular and binocular evoked average potential field topography: Upper and lower hemiretinal stimuli. Expl Brain Res 1983; 50: 341–6.
Skrandies W. Critical flicker fusion and double flash discrimination in different parts of the visual field. Intern J Neurosci 1985; 25: 225–31.
Skrandies W, Baier M. The standing potential of the human eye reflects differences between upper and lower retinal areas. Vision Res 1986; 26: 577–81.
Perry VH, Oehler R, Cowey A. Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience 1984; 12: 1101–23.
Perry VH, Cowey A. The ganglion cell and cone distributions in the monkey's retina: Implications for central magnification factors. Vision Res 1985; 25: 1795–810.
Reese BE, Cowey A. Segregation of functionally distinct axons in the monkey's optic tract. Nature 1988; 331: 350–4.
Salzman J, Seiple W, Carr R. Electrophysiological assessment of aphakic cystoid macular edema. Br J Ophthalmol 1986; 70: 719–24.
Shiroyama N, Miyake Y. Analysis of focal macular ERG in idiopathic central serous chorioretinopathy. Acta Soc Ophthalmol Jpn, in press.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Miyake, Y. Macular oscillatory potentials in humans. Doc Ophthalmol 75, 111–124 (1990). https://doi.org/10.1007/BF00146547
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00146547