Abstract
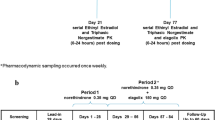
In a controlled clinical trial, the elimination of caffeine was examined in 20 healthy women prior to and during one cycle of treatment with either of two oral contraceptive formulations, one containing 0.075 mg gestodene and 0.03 mg ethinylestradiol and one containing 0.125 mg levonorgestrel and 0.03 mg ethinylestradiol. In addition, caffeine clearance was determined 1 month after the last intake of the oral contraceptives. Compared with pretreatment values, the clearance of caffeine was reduced by about 54% and 55% after one treatment cycle with gestodene- and the levonorgestrel-containing oral contraceptive, respectively. Other pharmacokinetic parameters of caffeine, such as t max and C max, were not affected. Clearance values returned to pretreatment values 1 month after the last administration of the oral contraceptives. There was no difference in the reduction of caffeine clearance between contraceptive formulations. A small, but significant difference in the AUC(0–24 h) values of ethinylestradiol was noted between both preparations. There was no correlation between the AUC(model) values of caffeine and the AUC(0–24 h) values of ethinylestradiol. In the present study, a somewhat more pronounced effect on the elimination of caffeine was observed than in previous investigations, where several contraceptive steroids were administered only for a period of 2 weeks.
Similar content being viewed by others
References
Fotherby K (1990) Interactions with oral contraceptives. Am J Obstet Gynecol 163:2153–2158
Meyer FP (1990) Einfluß hormonaler Kontrazeptiva auf die Kinetik anderer Pharmaka. Z Klin Med 45:1239–1241
Tornatore KM, Kanarkowski R, Carthy TL, Gardner MJ, Yurchak AM, Jusko WJ (1982) Effect of chronic oral contraceptive steroids on theophylline disposition. Eur J Clin Pharmacol 23:129–134
Rietveld EC, Broekman MMM, Houben JJG, Eskes TKAB, van Rossum JM (1984) Rapid onset of an increase in caffeine residence time in young women due to oral contraceptive steroids. Eur J Clin Pharmacol 26:371–373
Herz R, Koelz HR, Haemmerli UP, Benes I, Blum AL (1978) Inhibition of hepatic demethylation of aminopyrine by oral contraceptive steroids in humans. Eur J Clin Invest 8:27–30
Pazzucconi F, Malavasi B, Galli G, Franceschini G, Calabresi L, Sirtori CR (1991) Inhibition of antipyrine metabolism by low-dose contraceptives with gestodene and desogestrel. Clin Pharmacol Ther 49: 278–284
Böcker R, Kleingeist B, Eichhorn M, Lepper H (1991) In vitro interaction of contraceptive steroids with human liver cytochrome P-450 enzymes. Adv Contracept 7, [Suppl 3]:140–148
Back DJ, Houlgrave R, Tilja JF, Ward S, Orme LE (1991) Effect of the progestogens gestodene, 3-ketodesogestrel, levonorgestrel, norethisterone and norgestimate on the oxidation of ethinylestradiol and other substrates by human liver microsomes. J Steroid Biochem Mo Biol 38:219–225
Guengerich FP (1990) Mechanism-based inactivation of human liver microsomal cytochrome P-450 IIIA4 by gestodene. Chem Res Toxicol 3:363–371
Guengerich FP (1990) Inhibition of oral contraceptive steroid-metabolizing enzymes by steroids and drugs. Am J Obstet Gynecol 163:2159–2163
Balogh A, Irmisch E, Wolf P, Letrari S, Splinter FK, Hempel F, Klinger G, Hoffmann A (1991) Zum Einfluß von Levonorgestrel und Ethinylestradiol sowie deren Kombination auf die Aktivität von Biotransformationsreaktionen. Zentralbl Gynäkol 112:735–746
Eugster HP, Probst M, Würgler F, Sengstag C (1993) Caffeine, estradiol and progesterone interact with human CYP1A1 and CYP1A2. Evidence from cDNA-directed expression in Saccharomyces cerevisiae. Drug Metabol Dispos 21:43–49
Fuhr U, Doemer J, Battula N, Wolfel C, Flick I, Kudla C, Keita Y, Staib AH (1993) Biotransformation of methylxanthines in mammalian cell lines genetically engineered for expression of single cytochrome P-450 isoforms — allocation of metabolic pathways to isoforms and inhibitory effects of quinolones. Toxicology 82:1–3
Balogh A, Liewald T, Liewald S, Schröder S, Klinger G, Splinter FC, Hoffmann A (1990) Zum Einfluß eines neuen Gestagens — Dienogest — und seiner Kombination mit Ethinylestradiol auf die Aktivität von Biotransformationsreaktionen. Zentralbl Gynäkol 112:735–746
Kuhnz W, Louton T, Back DJ, Michaelis K (1993) Radioimmunological analysis of ethinylestradiol in human serum. Validation of the method and comparison with a gas chromatographic/mass spectrometric assay. Arzneimittelforschung 43:16–21
Balogh A, Harder S, Vollandt R, Staib H (1992) Intra-individual variability of caffeine elimination in healthy subjects. Int J Clin Pharmacol Ther Toxicol 30:383–387
Meyer FP, Walter H, Canzler E, Giers H (1988) Einfluß der oralen Kontrazeptiva Minisiston/Trisiston auf die Pharmakokinetik von Coffein — ein intraindividueller Langzeitvergleich. Z klin Med 44:239–240
Meyer FP, Canzler H, Giers H, Walter H (1988) Langzeituntersuchungen zum Einfluß von Non-Ovulon auf die Pharmakokinetik von Coffein im intraindividuellen Vergleich. Zentralbl Gynäkol 110:1449–1454
Jung-Hoffmann C, Kuhl H (1989) Interaction with the pharmacokinetics of ethinylestradiol and progestogens contained in oral contraceptives. Contraception 40:299–313
Orme M'LE, Back DJ, Ward S, Green S (1991) The pharmacokinetics of ethynylestradiol in the presence and absence of gestodene and desogestrel. Contraception 43:305–316
Kuhnz W, Back DJ, Power J, Schütt B, Louton T (1991) Concentration of ethinylestradiol in the serum of 31 young women following a treatment period of 3 months with two low-dose oral contraceptives in an intraindividual cross-over design. Horm Res 36:63–69
Dibbelt L, Knuppen R, Jütting G, Heimann S, Klipping CO, Parikka-Olexik H (1990) Group comparison of serum ethinyl estradiol, SHBG and CBG levels in 83 women using two low-dose combination oral contraceptives for three months. Contraception 43:1–21
Hammerstein J, Daume E, Simon A, Winkler UH, Schindler AE, Back DJ, Ward S, Neiss A (1993) Influence of gestodene and desogestrel as components of low-dose oral contraceptives on the pharmacokinetics of ethinyl estradiol (EE2), on serum CBG and on urinary cortisol and 6β-hydroxycortisol. Contraception 47:263–281
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Balogh, A., Henschel, L., Klinger, G. et al. Influence of ethinylestradiol-containing combination oral contraceptives with gestodene or levonorgestrel on caffeine elimination. Eur J Clin Pharmacol 48, 161–166 (1995). https://doi.org/10.1007/BF00192743
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00192743