Abstract
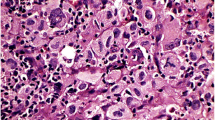
A total of 291 enlarged lymph nodes showing a range of reactive-inflammatory processes, primary and metastatic neoplasms were studied to determine the distribution and immunoprofile of their cytokeratin-positive interstitial reticulum cells (CIRC) in comparison with normal nodes. In 258/291 nodes (89%), CIRC numbers were distinctly increased in the subcapsular, paracortical and, occasionally, in the medullary zones; often, these increased CIRC formed networks around follicles, sinuses and vessels. CIRC had comparatively small, irregularly shaped bodies and dendritic processes; occasionally, giant forms were noted. CIRC contained cytokeratins (CK) 8 and 18 but not 19, as shown by immunohistochemistry, and by gel electrophoresis with subsequent immunoblotting. They co-expressed vimentin consistently, alpha-smooth-muscle actin frequently, and desmin less frequently. They did not contain desmoplakins, Factor VIII, S-100, LCA, B and T lymphocyte- and macrophage-associated antigens, chromogranin A, synaptophysin or the A-80 glycoprotein. We found no clear correlation between the increased CIRC and given nodal disease processes. However, CIRC were most abundant in nodes free of but draining malignant tumours; bizarre CIRC assemblies were noted in HIV lymphadenopathy. CIRC appear to represent a subset of the so-called “fibroblastic reticulum cells” of lymph nodes. Their function remains undetermined; their increase in diverse lymphadenopathies suggests that they partake in nodal reactions to injury. It remains unclear whether the increase in CIRC relative number is due to proliferation or to CK gene induction processes but their presence and potential capability to undergo hyperplasia with dysplastic forms should alert pathologists to possible diagnostic pitfalls. In addition, we discuss that CIRC may undergo transformation and represent the “cell of origin” of certain CK-positive tumours restricted to lymph nodes.
Similar content being viewed by others
References
Achtstaetter T, Hatzfeld M, Quinlan RA, Parmelee D, Franke WW (1986) Separation of cytokeratin polypeptides by gel electrophoresis and chromatographic techniques and their identification by immunoblotting. Methods Enzymol 134:355–371
Blobel GA, Moll R, Franke WW, Kayser KW, Gould VE (1985) The intermediate filament cytoskeleton of malignant mesotheliomas and its diagnostic significance. Am J Pathol 121:235–247
Bolen JW, Hammar SP, McNutt MA (1987) Serosal tissue: reactive tissue as a model for understanding mesotheliomas. Ultrastruct Pathol 11:251–262
Brandtzaeg P, Jones DB, Flavell DJ, Fagerhol MK (1988) Monoclonal antibody to a human macrophage marker (Mac 387) detects the formalin resistant myelomonocytic L1 antigen. J Clin Pathol 41:963–970
Burke AP, Anderson D, Mannan P, Ribas JL, Liang Y-H, Smialek J, Virmani R (1994) Systemic lymphadenopathy histology in human immunodeficiency virus-1 seropositive drug addicts without apparent acquired immunodeficiency syndrome. Hum Pathol 25:248–256
Coggi G, Dell'Orto P, Braidotti P, Coggi A, Viale G (1989) Coexpression of intermediate filaments in normal and neoplastic human tissues. Ultrastruct Pathol 13:501–514
de Mascarel A, Merlio JP, Coindre JM, Goussot JF, Broustet A (1989) Gastric large cell lymphoma expressing cytokeratin but no leucocyte common antigen. Am J Clin Pathol 91:478–481
Doglioni C, Dell'Orto P, Zanetti G, Iuzzolino P, Coggi G, Viale G (1990) Cytokeratin immunoreactive cells of human lymph nodes and spleen in normal and pathological conditions. Virchows Arch [A] 41:479–490
Eusebi V, Capella C, Cossu A, Rosai J (1992) Neuroendocrine carcinoma within lymph nodes in the absence of a primary tumor with special reference to Merkel cell carcinoma. Am J Surg Pathol 16:658–666
Franke WW, Moll R (1987) Cytoskeletal components of lymphoid organs. I. Synthesis of cytokeratins 8 and 18 and desmin in subpopulations of extrafollicular reticulum cells of human lymph nodes, tonsils and spleen. Differentiation 36:145–163
Franke WW, Jahn L, Knapp AC (1989) Cytokeratins and desmosomsal proteins in certain epithelioid and non-epithelial cells. In: Osborn M, Weber K (eds) Current communications in molecular biology, cytoskeletal proteins in tumor diagnosis, Cold Spring Harbor, NY, Cold Spring Harbour Laboratory, pp 151–172
Gould VE, Wiedenmann B, Lee I, Schwechheimer K, Dockhorn-Dworniczak B, Radosevich JA, Moll R, Franke WW (1987) Synaptophysin expression in neuroendocrine neoplasms as determined by immunocytochemistry. Am J Pathol 126:243–257
Gould VE, Shin SS, Manderino GL, Rittenhouse HG, Tomita JT, Gooch GT (1988) Selective expression of novel mucintype glycoprotein in human tumors. Immunohistochemical demonstration with Mab A-80. Hum Pathol 19:623–627
Gould VE, Warren WH, Faber LP, Kuhn C, Franke WW (1990) Malignant cells of epithelial phenotype limited to thoracic lymph nodes. Eur J Cancer 26:1121–1126
Gould VE, Koukoulis GK, Jansson DS, Nagle RB, Franke WW, Moll R (1990) Coexpression patterns of vimentin and glial filament protein with cytokeratins in the normal hyperplastic and neoplastic breast. Am J Pathol 137:1143–1155
Gustmann C, Altmannsberger M, Osborn M, Griesser H, Feller AC (1991) Cytokeratin expression and vimentin content in large cell anaplastic lymphomas and other non-Hodgkin's lymphomas. Am J Pathol 138:1413–1422
Juzzolino P, Bontempine L, Doglioni C, Zanetti G (1989) Keratin immunoreactivity in extrafollicular reticular cells of the lymph node (letter). Am J Clin Pathol 91:239–240
Jahn L, Fouquet B, Rohe K, Franke WW (1987) Cytokeratins in certain endothelial and smooth muscle cells of two taxonomically distant species, Xenopus levis and man. Differentiation 36:234–254
Jahn L, Kruezer J, von Hodenberg E, Kuebler W, Franke WW, Allenberg J, Izumo S (1993) Cytokeratins 8 and 18 in smooth muscle cells. Detection in human coronary artery, peripheral vascular and vein graft disease and in transplantation-associated arteriosclerosis. Arteriosclerosis Thrombosis 13:1631–1639
Jansson DS, Gould VE, Gooch GT, Rittenhouse HG, Shin SS, Manderino GL, Tomita JT, Staren ED (1988) Immunohistochemical analysis of colon carcinomas applying exocrine and neuroendocrine markers. APMIS 96:1129–1139
Knapp AC, Franke WW (1989) Spontaneous losses of control of cytokeratin gene expression in transformed, non-epithelial human cells occurring at different levels of regulation. Cell 59:67–79
Knapp AC, Bosch FX, Hergt M, Kuhn C, Winter-Simanowski S, Schmitt E, Regauer S, Bartek J, Franke WW (1989) Cytokeratins and cytokeratin filaments in subpopulations of cultured human and rodent cells of nonepithelial origin: modes and pattern of expression. Differentiation 42:81–102
Knowles DM, Chardburn A (1992) Lymphadenopathy and the lymphoid neoplasms associated with the acquired immune dificiency syndrome (AIDS). In: Knowles DM (ed) Neoplastic hematopathology, Wilson and Wilkins, Baltimore, pp. 773–835
Koukoulis GK, Shin SS, Gould VE, Jao W, Gooch GT, Manderino GL, Rittenhouse HG, Tomita JT (1990) Immunohistochemical evaluation of neoplastic and non-neoplastic breast diseases with Mab A-80. Pathol Res Pract 186:439–449
Koukoulis GK, Radosevich JA, Warren WH, Rosen ST, Gould VE (1990) Immunohistochemical analysis of pulmonary and pleural neoplasms with monoclonal antibodies B72.3 and CSLEX-1. Virchows Arch [B] 58:427–433
Miettinen M, Virtanen I, Talerman A (1985) Intermediate filament proteins in human testis and testicular germ-cell tumors. Am J Pathol 120:402–410
Moll R, Franke WW, Schiller DL, Geiger B, Krepler R (1982) The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 31:11–24
Moll R (1989) Cytoskeletal markers in the classification of carcinomas and their metastases. In: Osborn M, Weber K (eds) Current communications in molecular biology: cytoskeletal proteins in tumor diagnosis. Cold Spring Harbor Laboratory, Cold spring Harbor, New York, pp 139–144
Moll R (1993) Cytokeratine als Differenzierungsmarker: Expressionsprofile von Epithelien und epithelialen Tumoren. Gustav Fischer Verlag, Stuttgart
Moll R, Krepler R, Franke WW (1983) Complex cytokeratin polypeptide patterns observed in certain human carcinomas. Differentiation 23:256–269
Moll R, Cowin P, Kaprell H-P, Franke WW (1986) Desmosomal proteins: new markers for identification and classification of tumors. Lab Invest 54:4–25
Moll R, Dhouailly D, Sun TT (1989) Expression of keratin 5 as a distinctive feature of epithelial and biphasic mesotheliomas: an immunohistochemical study using monoclonal antibody AE14. Virchows Arch [B] 59:129–145
Moll R, Zimbelmann R, Goldschmidt MD, Keith M, Laufer J, Kasper M, Koch PJ, Franke WW (1993) The human gene encoding cytokeratin 20 and its expression during fetal development and in gastrointestinal carcinoma. Differentiation 53:75–93
Nathwani BN (1992) Diagnostic significance of morpholic patterns in lymph node proliferations. In: Knowles DM (ed) Neoplastic hematopathology, Williams and Wilkins, Baltimore, pp. 407–425
Pinkus GS, Warhol MJ, O'Connor EM, Etheridge CL, Fujuwara K (1986) Immunohistochemical localization of smooth muscle myosin in human spleen, lymph node and other lymphoid tissues: unique staining patterns in splenic white pulp and sinuses, lymphoid follicles, and certain vasculature, with ultrastructural correlations. Am J Pathol 123:440–453
Schmelz M, Franke WW (1993) Complexus adherents, a new group of desmoplakin containing junctions in endothelial cells: the syndesmosomes connecting retothelial cells of lymph nodes. Eur J Cell Biol 61:274–289
Schmelz M, Moll R, Kuhn C, Franke WW (1994) Complexus adhaerentes, a new group of desmoplakin-containing junctions in endothelial cells. II. Different types of lymphatic vessels. Differentiation 578:97–117
Schnitzer B (1992) Reactive lamphadenopathies. In: Knowles DM (ed) Neoplastic hematopathology, Wiliams and Wilkins, Baltimore, pp 427–457
Shi S-R, Key ME, Kalra KL (1991) Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement methods for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 39:741–748
Shin SS, Gould VE, Gould JE, Warren WH, Gould KA, Yaremko L, Manderino GL, Rittenhouse HG, Tomita JT, Jansson DS, Staren ED, Chejfec G, Gooch GT (1989) Expression of a new mucin-type glycoprotein in select epithelial dysplasias and neoplasms detected immunocytochemically with monoclonal antibody A-80. APMIS 97:1053–1067
Stosiek P, Kasper M, Karsten U (1990) Expression of cytokeratins 8 and 18 in human Sertoli cells of immature and atrophic seminiferous tubules. Differentiation 43:66–70
Swanson PE, Strickler JG (1989) Gastric lymphoma expressing cytokeratin but no leucocyte common antigen: an editorial dilemma (letter). Am J Clin Pathol 91:707
Toccanier-Pelte MF, Skalli O, Kapanci Y, Gabbiani G (1987) Characterization of stromal cells with myoid features in lymph nodes and spleen in normal and pathologic conditions. Am J Pathol 129:109–118
van der Valk P, Miejr CJLM (1987) The histology of reactive lymph nodes. Am J Surg Pathol 11:866–882
Viale G (1989) Cytokeratin immunochemistry in the practice of diagnostic pathology. Ultrastruct Pathol 13:91 (lett to the ed)
Wiedenmann B, Franke WW, Moll R, Kuhn C, Gould VE (1986) Synaptophysin: a marker protein for neuroendocrine cells and neoplasms. Proc Natl Acad Sci USA 83:3500–3504
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gould, V.E., Bloom, K.J., Franke, W.W. et al. Increased numbers of cytokeratin-positive interstitial reticulum cells (CIRC) in reactive, inflammatory and neoplastic lymphadenopathies: hyperplasia or induced expression?. Vichows Archiv A Pathol Anat 425, 617–629 (1995). https://doi.org/10.1007/BF00199352
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00199352