Summary
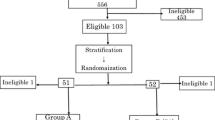
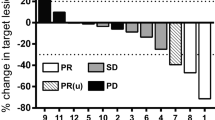
A phase I study was carried out to test the feasibility and toxicity of infusing large numbers of autologous, alloactivated helper lymphocytes into patients with metastatic melanoma. Patient peripheral blood lymphocytes (Pt-PBL) obtained by lymphopheresis and expressing the helper phenotype BT5/9 were separated and stimulated for 48 or 72 h with a pool of PBL from four to six healthy donors. Patients were then infused with such activated lymphocytes over a 2–3 h period. A total of 4 phereses and infusions (2/week for 2 weeks) were carried out for each cycle in each patient. Of the five patients treated, two received a second round of infusions. Infusion of autologous PBL stimulated in vitro for 48 h caused chills, fever, headache, and increased blood pressure. All symptoms disappeared in 2–3 h and were easily controlled by appropriate therapy. When lymphocytes were given after 72 h of allostimulation, no or very mild toxicity was observed. Serum chemistry, coagulation, autoimmunity, and urine analysis showed no gross abnormalities during therapy or follow-up of the patients. Immunological parameters (OKT4/OKT8 ratio, NK activity and cytotoxic T cell activity to autologous melanoma) were evaluated before starting the therapy, during its course and during the 3 to 6 months follow-up. The OKT4/OKT8 ratio increased significantly but transiently soon after the first course of infusions in one of the two patients tested. NK activity increased after 75–100 days in the three patients tested and in one of them it was high even after 180 days. No correlation between NK activity and prognosis was apparent. Cytotoxicity to autologous tumor was assessed in two patients, only one of whom exhibited an increased activity from 75 to 180 days, which was associated with a prognosis better than that of the negative patient. Five patients were treated: two had progressive disease, two had stable disease for 5 and 6 months, respectively. In the first of these patients, a new cycle of lymphocyte infusions was carried out which caused a measurable reduction of lung tumor nodules whose growth, however, resumed 4 months later. This patient died 14 months after the onset of therapy. The fifth patient had a partial regression of pulmonary and intracranial metastases after therapy, but eventually died 3 months later. These results indicate that infusion of a high numbers of autologous, allostimulated helper PBL is a feasible and safe procedure, which could therefore be used in future studies of adoptive immunotherapy of cancer.
Similar content being viewed by others
References
Balch CM, Tilden AB, Dougherty PA, Cloud GA, Torju ABO (1983) Depressed levels of granular lymphocytes with natural killer (NK) cell function in 247 cancer patients. Ann Surg 198:192
Balsari A, Fossati G, Taramelli D, Nava M, Ravagnani F, Parmiani G (1984) Inhibition of human melanoma growth in nude mice by autologous, alloactivated peripheral blood lymphocytes. Tumori 70:35
Balsari A, Fossati G, Taramelli D, Tona G, Delia D, Giardini R, Parmiani G (1985) Allostimulation of patients'lymphocytes generates both T and NK-like cells cytotoxic for autologous melanoma. Br J Cancer 52:73
Carter SK (1984) Perusing the ASCO abstracts with a focus on interferons in malignant melanoma and renal cell carcinoma. Cancer Chemother Pharmacol 13:153
Colombo MP, Arioli I, Parmiani G (1984) Passive adoptive immunotherapy of a low immunogenic BALB/c lymphoma by syngeneic alloimmune T lymphocytes. Int J Cancer 34:807
Corte G, Mingari MC, Moretta A, Damiani G, Moretta L, Bargellesi A (1982) Human T cell subpopulations defined by a monoclonal antibody. I. A small subset is responsible for proliferation to allogeneic cells or to soluble antigens and for helper activity for E+ cell differentiation. J Immunol 128:16
Creagan ET, Ahmam DL, Green SJ, Long HJ, Frytek S, O'Fallon JR, Itri LM (1984) Phase II study of low dose recombinant leukocyte A interferon in disseminated malignant melanoma. J Clin Oncol 2:1002
Dillman RO, Koziol JA, Zavanelli MI, Beauregard JC, Halliburton BL, Glassy M, Royston I (1984) Immunocompetence in cancer patients. Assessment by in vitro stimulation tests and quantification of lymphocyte subpopulations. Cancer 53:1484
Dye ES, North RJ (1981) T cell-mediated immunosuppression as an obstacle to adoptive immunotherapy of the P815 mastocytoma and its metastases. J Exp Med 154:1033
Einhorn S, Blomgren H, Strander H, Wasserman J (1983) Influence of human interferon-α therapy on cytotoxic functions of blood lymphocytes. Study on lectin-dependent cellular cytotoxicity, antibody-dependent cellular cytotoxicity, and natural killer cell activity. Cancer Immunol Immunother 16:77
Fefer A, Goldstein AL (1982) The potential role of T cells in cancer therapy. Raven Press, New York
Fernandez-Cruz E, Gilman SC, Feldman JD (1982) Immunotherapy of a chemically-induced sarcoma in rats: characterization of the effector T-cell subset and nature of suppression. J Immunol 128:112
Fossati G, Balsari A, Taramelli D, Sensi ML, Pellegris G, Nava M, Parmiani G (1982) Lysis of autologous human melanoma cells by in vitro allosensitized peripheral blood lymphocytes. Cancer Immunol Immunother 14:99
Fossati G, Taramelli D, Balsari A, Bogdanovich G, Andreola S, Parmiani G (1984) Primary but not metastatic melanomas expressing DR antigens stimulate autologous lymphocytes. Int J Cancer 33:591
Fujiwara H, Fukurawa M, Yoshioka T, Nakajima H, Hamaoka T (1984) The role of tumor-specific Lyt 1 + 2-T cells in eradicating tumor cells in vivo.I.Lyt1 + 2-T cells do not necessarily require recruitment of host's cytotoxic T cell precursors for implementation of in vivo immunity. J Immunol 133:1671
Golub SH, Dorey F, Hara D, Morton DL, Burk MW (1982) Systemic administration of human leukocyte interferon to melanoma patients. I. Effects on natural killer function and cell pupulations. J Natl Cancer Inst 68:703
Greenberg PD, Cheever MA, Fefer A (1981) Eradication of disseminated murine leukemia by chemoimmunotherapy with cyclophosphamide and adoptively transferred immune syngeneic Lyt 1 + 2- lymphocytes. J Exp Med 154:952
Guerry D IV, Alexander MA, Herlyn MF, Zehngebot LM, Mitchell KF, Zmijewski CM, Lusk EJ (1984) HLA-DR histocompatibility leukocyte antigens permit cultured human melanoma cells from early but not advanced disease to stimulate autologous lymphocytes. J Clin Invest 73:267
Kaszubowski PW, Husby G, Tung KSK, Williams RC (1980) T-lymphocyte subpopulation in peripheral blood and tissues of cancer patients. Cancer Res 40:4648
Ling MR, Bishop S, Jefferis R (1977) Use of antibody-coated red cells for the sensitive detection of antigen and in rosette tests for cells bearing surface immunoglobulins. J Immunol Methods 15:279
Mazumder A, Rosenberg SA (1984) Successful immunotherapy of natural killer-resistant established pulmonary melanoma metastases by the intravenous adoptive transfer of syngeneic lymphocytes activated in vitro by interleukin 2. J Exp Med 15:495
Mazumder A, Eberlein TJ, Grimm EA, Wilson DJ, Keenan AM, Aamodt R, Rosenberg SA (1984) Phase I study of adoptive immunotherapy of cancer with lectin-activated autologous mononuclear cells. Cancer 53:896
Mills CD, North RJ (1983) Expression of passively transferred immunity against established tumors depends on generation of cytolytic T cells in recipient. Inhibition by suppressor T cells. J Exp Med 157:1448
Mulé J, Shu S, Schwarz S, Rosenberg SA (1984) Adoptive immunotherapy of established pulmonary metastasis with LAK cells and recombinant interleukin-2. Science 225:1487
Oppenheim JJ, Stadler BM, Siraganian RP, Mage M, Mathieson B (1982) Lymphokines: their role in lymphocyte responses. Properties of interleukin 1. Fed Proc 41:257
Paciucci PA, MacPhail S, Zarling JM, Bach FH (1980) Lysis of syngeneic tumor cells by alloantigens stimulates mouse T and non-T cells. J Immunol 24:370
Peterman GM, Stanton GJ, Altman LC, Khimper GR (1984) Interferon production and tumor cell killing by human lymphocytes stimulated in mixed-lymphocyte culture. Cell Immunol 85:114
Rosestein M, Yron N, Kaufmann Y, Rosenberg SA (1984) Lymphokine-activated killer cells: lysis of fresh syngeneic natural killer-resistant murine tumor cells by lymphocytes cultured in interleukin 2. Cancer Res 44:1946
Scala G, Allavena P, Djeu J, Kasahara T, Ortaldo JR, Herberman RB, Oppenheim JJ (1984) Human large granular lymphocytes are potent producers of interleukin-1. Nature 309:56
Schmidt JA, Mizel SB, Cohen D, Green I (1982) Interleukin 1, a potential regulator of fibroblasts proliferation. J Immunol 128:2177
Sensi ML, Parenza M, Parmiani G (1983) Alloreactivity and tumor antigens: Generation of syngeneic antilymphoma killer lymphocytes by allostimulation of mice with normal cells. J Natl Cancer Inst 70:291
Spitler L, Sagibiel R (1980) A randomized trial of levamisole versus placebo as adjuvant therapy in malignant melanoma. N Engl J Med 303:1143
Strausser JL, Mazumder A, Grimm EA, Lotze MT, Rosenberg SA (1981) Lysis of human solid tumors by autologous cells sensitized in vitro to alloantigens. J Immunol 127:266
Trinchieri G, Perussia B (1984) Human natural killer cells: Biologic and pathologic aspects. Lab Invest 50:489
Vanky F, Gorsky T, Gorsky Y, Masucci MG, Klein E (1982) Lysis of tumor biopsy cells by autologous T lymphocytes activated in mixed cultures and propagated with T cell growth factor. J Exp Med 155:83
Vanky F, Peterffy A, Böök K, Williams J, Klein E, Klein G (1983) Correlation between lymphocyte-mediated anti-tumor reactivities and clinical course. II. Evaluation of 69 patients with lung carcinoma. Cancer Immunol Immunother 16:17
Vanky F, Williams J, Kreicbergs A, Parisi T, Andreen M, Brostrom L, Nilsonne U, Klein E, Klein G (1983) Correlation between lymphocyte-mediated auto-tumor reactivities and clinical course. I. Evaluation of 46 patients with sarcoma. Cancer Immunol Immunother 16:11
Veronesi U, Adamus J, Aubert C, Bajetta E, Beretta G, Bonadonna G, Bufalino R, Cascinelli N, Cocconi G, Durand J, De Marsillac J, Ikonopisov RL, Kiss B, Lejeune F, MacKie R, Madej G, Mulder H, Mechl Z, Milton GW, Morabito A, Peter H, Priario J, Paul E, Rumke P, Sertoli R, Tomin R (1982) A randomized trial of adjuvant chemotherapy and immunotherapy in cutaneous melanoma. N Engl J Med 307:913
Vose BM, White W (1983) Tumor-reactive lymphocytes stimulated in mixed lymphocyte and tumor culture. Cancer Immunol Immunother 15:227
Zarling JM, Robins HI, Raich PC, Bach FH, Bach ML (1978) Generation of cytotoxic lymphocytes to autologous human leukemia cells by sensitization of pooled allogeneic normal cells. Nature 274:269
Zarling JM, Kay NE, Grant B, Yasukawa M, Bach FH (1983) Human anti-lymphoma responses generated in vitro and in vivo following sensitization with allogeneic leukocytes. Cancer Immunol Immunother 15:237
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Balsari, A., Marolda, R., Gambacorti-Passerini, C. et al. Systemic administration of autologous, alloactivated helper-enriched lymphocytes to patients with metastatic melanoma of the lung. Cancer Immunol Immunother 21, 148–155 (1986). https://doi.org/10.1007/BF00199863
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00199863