Summary
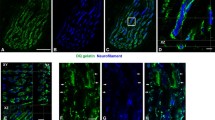
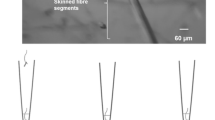
The in-vivo uptake of exogenously applied horseradish peroxidase and the activities of the lysosomal enzymes acid phosphatase and cathepsin D were studied histochemically and/or biochemically in innervated and 2–14 day-denervated tibialis anterior muscles of the mouse. The biochemically determined uptake of horseradish peroxidase showed a large increase already 4 days after denervation. The activities of the lysosomal enzymes increased in a more gradual fashion, and only cathepsin D showed an increase in activity when expressed as total activity per muscle. Histochemically horseradish peroxidase was found to be localized in muscle fibres in characteristic spindle-shaped segments after denervation. The main increase in the number of such segments per transverse section of the muscle occurred between 3 and 6 days after denervation. In serial sections these segments frequently showed positive staining also for acid phosphatase.
It is concluded that exogenously applied horseradish peroxidase is taken up into the lysosomal system, which after denervation becomes organized into characteristic spindle-shaped segments in the muscle fibres. The endocytic activity of muscle fibres increases early after denervation. This is followed by a more gradual increase in activity of lysosomal enzymes and finally by an organization of the lysosomal system into characteristic spindle-shaped segments. The results are compatible with the working hypothesis that increased endocytosis may initiate lysosomal activation in denervated skeletal muscle.
Similar content being viewed by others
References
Barka T (1960) A simple azo-dye method for histochemical demonstration of acid phosphatase. Nature 187:248–249
Barka T, Anderson PJ (1962) Histochemical methods for acid phosphatase using hexazonium pararosanilin as coupler. J Histochem Cytochem 10:741–753
Barrett AJ, Heath MF (1977) Lysosomal enzymes. In: Dingle JT (ed) Lysosomes, a laboratory handbook. 2nd edition. North Holland, Amsterdam, p 124
Bird JWC (1975) Skeletal muscle lysosomes. In: Dingle JT, Dean RT (eds) Lysosomes in biology and pathology, Vol 4. North Holland, Amsterdam, p 75
Boegman RJ, Scarth B (1981) Response of skeletal muscle to neural application of batrachotoxin or tetrodotoxin: Endocytosis of extracellular markers. Exp Neurol 74:855–861
Burstone MS (1958) Histochemical demonstration of acid phosphatases with naphtol AS-phosphates. J Natl Cancer Inst 21:523–540
Canonico PG, Bird JWC (1970) Lysosomes in skeletal muscle tissue. Zonal centrifugation evidence for multiple cellular sources. J Cell Biol 45:321–333
Graham RC, Karnovsky MJ (1966) The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: Ultrastructural cytochemistry by a new technique. J Histochem Cytochem 14:291–302
Libelius R, Jirmanová I, Lundquist I, Thesleff S (1978a) Increased endocytosis with lysosomal activation in skeletal muscle of dystrophic mouse. J Neuropathol Exp Neurol 37:387–400
Libelius R, Lundquist I, Templeton W, Thesleff S (1978b) Intracellular uptake and degradation of extracellular tracers in mouse skeletal muscle in vitro: The effect of denervation. Neuroscience 3:641–647
Libelius R, Jirmanová I, Lundquist I, Thesleff S, Barnard EA (1979a) T-tubule endocytosis in dystrophic chicken muscle and its relation to muscle fiber degeneration. Acta Neuropathol (Berl) 48:31–38
Libelius R, Josefsson J-O, Lundquist I (1979b) Endocytosis in chronically denervated mouse skeletal muscle. A biochemical and ultrastructural study with horseradish peroxidase. Neuroscience 4:283–292
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lundquist I, Josefsson J-O (1971) Sensitive method for determination of peroxidase activity in tissue by means of coupled oxidation reaction. Analyt Biochem 41:567–577
Meijer AEFH, Israel DE, van der Loos C, Tigges AJ (1979) Evaluation of histochemical observations of activity of acid hydrolases obtained with semipermeable membrane techniques. 3. The substrate specificity of isoenzymes of acid phosphatase in m. gastrocnemius of rabbits. Histochemistry 60:145–153
Meijer AEFH, van der Loos CM, Schuurhuizen PW (1980) The presence of a low molecular weight acid phosphatase in liver tissue that cannot be demonstrated with the histochemical substrate naphtol AS-BI phosphate. Histochemistry 67:23–29
Rowland LP (1980) Biochemistry of muscle membranes in Duchenne muscular dystrophy. Muscle Nerve 3:3–20
Sellin LC, Libelius R, Lundquist I, Tågerud S, Thesleff S (1980) Membrane and biochemical alterations after denervation and during reinnervation of mouse skeletal muscle. Acta Physiol Scand 110:181–186
Tachikawa T, Clementi F (1979) Early effects of denervation on the morphology of junctional and extrajunctional sarcolemma. Neuroscience 4:437–451
Thesleff S (1974) Physiological effects of denervation of muscle. Ann NY Acad Sci 228:89–104
Thesleff S, Libelius R, Lundquist I (1979) Endocytosis as inducer of degenerative changes in skeletal muscle. In: Kidman AD, Tomkins JK (eds) Muscle, nerve and brain degeneration. Excerpta Medica, Amsterdam, p 119
Weinstock IM, Iodice AA (1969) Acid hydrolase activity in muscular dystrophy and denervation atrophy. In: Dingle JT, Fell HB (eds) Lysosomes in biology and pathology, Vol 1. North-Holland, Amsterdam, p 450
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tågerud, S., Libelius, R. Lysosomes in skeletal muscle following denervation. Cell Tissue Res. 236, 73–79 (1984). https://doi.org/10.1007/BF00216515
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00216515