Summary
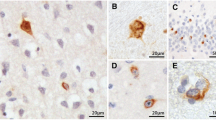
According to the literature, only minor nonspecific histopathological lesions are present in the motor cortex in up to 90% of the amyotrophic lateral sclerosis (ALS) patients. These observations, however, have so far been based mainly on conventional staining techniques. An exception to this is the focal glial reaction that has been reported following immunocytochemical staining for glial fibrillary protein (GFAP), which is reported to be distinctive for ALS in the cortex. Since perikarya of degenerating motor neurons in the spinal cord of ALS patients have been found to accumulate phosphorylated neurofilaments (PNF), an investigation was conducted to determine whether PNF was also a sensitive marker for alterations in the motor cortex in this condition. On large brain sections from 15 ALS patients, intense PNF immunoreactivity was found in the motor cortex from 11 patients. It was mainly localized in small pyramidal cells and basket cells, whereas only slight staining was observed in Betz cells. PNF-positive basket cells were also present in controls, but the basket cells staining for PNF were less numerous in controls than in ALS specimens. PNF-positive Betz cells were found in 47% of 15 ALS patients and in 10% of the controls. PNF accumulation was also found in swollen, probably degenerating, terminal boutons around perikarya of large pyramidal cells and Betz cells in the motor areas of ALS patients only. These observations suggest that the premotor innervation of the motor system is preferentially affected in ALS. Small brain sections, comprising the motor cortex, from 18 additional ALS patients demonstrated a similar PNF-staining pattern. However, differentiating ALS patients from controls was much easier when studying large brain sections. No ubiquitin-immunoreactive inclusions were found, except for sporadic tangles. The presence of a focal-GFAP positive astrocytosis as reported in the literature in the precentral cortex was confirmed. However, it was found to be nonspecific since it was also present outside the precentral cortex and in the cortex of normal control patients. No spatial relation was found between the distribution of the glial relation in ALS and the areas containing neurons and boutons accumulating PNF.
Similar content being viewed by others
References
Bertrand I, van Bogaert L, (1925) Rapport sur la sclérose latérale amyotrophique. Rev Neurol (Paris) 32:779–806
Bodian D (1971) Presynaptic organelles and junctional integrity. J Cell Biol 48:707–711
Braak H (1984) Architectonics as seen by lipofuscin stains. In: Peters A, Jones EG (eds) Cerebral cortex, cellular components of the cerebral cortex, vol 1. Plenum Press, New York, pp 59–104
Brownell B, Oppenheimer DR, Hughes JT (1970) The central nervous system in motoneurone disease. J Neurol Neurosurg Psychiatry 33:338–357
Carpenter S (1968) Proximal axonal enlargement in motor neuron disease. Neurology 18:841–851
Castaigne P, Lhermitte F, Cambier J, Escourolle R, Le Bigot P (1972) Etude neuropathologique de 61 observations de sclérose latérale amyotrophique. Rev Neurol (Paris) 127:401–414
Colmant HJ (1988) Die myatrophische Lateralsklerose. In: Lubarsch O, Henke F, Rössle (eds) Handbuch der speziellen pathologischen Anatomie und Histologie, vol 13. Springer-Verlag Berlin Göttingen Heidelberg, pp 2624–2692
Cork LC, Sternberger NH, Sternberger LA, Casanova MF, Struble RG, Price DL (1986) Phosphorylated neurofilament antigens in neurofibrillary tangles in Alzheimer's disease. J Neuropathol Exp Neurol 45:56–64
Goldman JE (1983) The association of actin with Hirano bodies. J Neuropathol Exp Neurol 42:146–152
Goldman JE, Yen S-H (1986) Cytoskeletal protein abnormalities in neurodegenerative diseases. Ann Neurol 19:209–223
Graham RC, Karnovsky MJ (1966) The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse: ultrastructural cytochemistry by a new technique. J Histochem Cytochem 4:291–302
Gray EG, Guillery (1966) Synaptic morphology in the normal and degenerating nervous system. Int Rev Cytol 19:111–182
Hirano A, Iwata M (1979) Pathology of motor neurons with special reference to amyotrophic lateral sclerosis and related diseases. In: Tsubaki T, Toyokura U (eds) Amyotrophic lateral sclereosis. University Park Press, Baltimore, pp 107–133
Jones EG, Hendry SHC (1984) Basket cells. In: Peters A, Jones EG (eds) Cerebral cortex, cellular components of the cerebral cortex, vol 1. Plenum Press, New York, pp 309–336
Kamo H, Haebara H, Akiguchi I, Kameyama M, Kimura H, McGeer PL (1987) A distinctive distribution of reactive astroglia in the precentral cortex in amyotrophic lateral sclerosis. Acta Neuropathol (Berl) 74:33–38
Kusaka H, Hirano A (1985) Fine structure of anterior horns in patients without amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 44:430–438
Kushner PD, Stephenson DT, Wright BS (1991) Reactive astrogliosis is widespread in the subcortical white matter of amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 50:263–277
Langley OK, Sternberger NH, Sternberger LA (1988) Expression of neurofilament proteins by Purkinje cells: ultrastructural immunolocalization with monoclonal antibodies. Brain Res 457:12–20
Lawyer T, Netsky MG (1953) Amyotrophic lateral sclerosis. Arch Neurol Psychiatry 69:171–192
Leigh PN, Swash M (1991) Cytoskeletal pathology in motor neuron disease. Adv Neurol 56:115–125
Leigh PN, Dodson A, Swash M, Brion J-P, Anderton BH (1989) Cytoskeletal abnormalities in motor neuron disease. An immunocytochemical study. Brain 112:521–535
Lowe J, Blanchard A, Morrell K et al (1988) Ubiquitin is a common factor in intermediate filament inclusion bodies of diverse type in man, including those of Parkinson's disease, as well as Rosenthal fibres in cerebellar astrocytomas, cytoplasmic bodies in muscle, and Mallory bodies in alcoholic liver disease. J Pathol 155:9–15
Lowe J, Aldridge F, Lennox G, Doherty F, Jefferson D, Landon M, Mayer RJ (1989) Inclusion bodies in motor cortex and brainstem of patients with motor neurone disease are detected by immunocytochemical localization of ubiquitin. Neurosci Lett 105:7–13
Malamud N (1968) Neuromuscular system disease. In: Minckler I (ed) Pathology of the nervous system. McGraw-Hill, New York, pp 712–730
Manetto V, Sternberger NH, Perry G, Sternberger LA, Gambetti P (1988) Phosphorylation of neurofilaments is altered in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 47:642–653
Marin-Padilla M (1969) Origin of the pericellular baskets of the pyramidal cells of the human motor cortex: a Golgi study. Brain Res 14:633–646
Mizusawa H, Matsumoto S, Yen S-H, Hirano A, Rojas-Corona RR, Donnenfeld H (1989) Focal accumulation of phosphorylated neurofilaments within anterior horn cell in familial amyotrophic lateral sclerosis. Acta Neuropathol 79:37–43
Munoz DG, Greene C, Perl DP, Selkoe DJ (1988) Accumulation of phosphorylated neurofilaments in anterior horn motoneurons of amyotrophic lateral sclerosis patients. J Neuropathol Exp Neurol 47:9–18
Murayama S, Mori H, Ihara Y, Bouldin TW, Suzuki K, Tomonaga M (1990) Immunocytochemical and ultrastructural studies of lower motor neurons in amyotrophic lateral sclerosis. Ann Neurol 27:137–148
Murayama S, Inoue K, Kawakami H, Bouldin TW, Suzuke K (1991) A unique pattern of astrocytosis in the primary motor area in amyotrophic lateral sclerosis. Acta Neuropathol 82:456–461
Nukina N (1989) The reinterpretation of the immunochemical study of Alzheimer neurofibrillary tangles. Ann Med 21:117–119
Nukina N, Kosik KS, Selkoe DJ (1987) Recognition of Alzheimer paired helical filaments by monoclonal neurofilament antibodies is due to cross reaction with tau protein. Proc Natl Acad Sci USA 84:3415–3419
Okamoto K, Hirai S, Yamazaki T, Sun X, Nakazato Y (1991) New ubiquitin-positive intraneuronal inclusions in the extramotor cortices in patients with amyotrophic lateral sclerosis. Neurosci Lett 129:233–236
Perry G, Stewart D, Friedeman R, Manetto V, Autilio-Gambetti L, Gambetti P (1987) Filaments of Pick's bodies contain altered cytoskeletal elements. Am J Pathol 127:559–568
Rechsteiner M (1987) Ubiquitin-mediated pathways for intracellular proteolysis. Annu Rev Cell Biol 3:1–30
Robinson PA, Anderton BH (1988) Neurofilament Probes — A review of neurofilament distribution and biology. Rev Neurosci 2:1–40
Schiffer D, Autilio-Gambetti L, Chio A, Gambetti P, Giordana MT, Gullotta F, Migheli A, Vigliani MC (1991) Ubiquitin in motor neuron disease: study at the light and electron microscope. J Neuropathol Exp Neurol; 50:463–473
Schröder P (1910) Über Hirnrindenveränderungen bei amyotrophischer Lateralsklerose. J Psychol Neurol 16:60–78
Sobue G, Hashizume Y, Yasuda T, Mukai E, Kumagai T, Mitsuma T, Trojanowski JQ (1990) Phosphorylated high molecular weight neurofilament protein in lower motor neurons in amyotrophic lateral sclerosis and other neurodegenerative diseases involving ventral horn cells. Acta Neuropathol 79:402–408
Sternberger NH, Sternberger LA, Ulrich J (1985) Aberrant neurofilament phosphorylation in Alzheimer disease. Proc Natl Acad Sci USA 82:4274–4276
Wohlfahrt S (1932) Die vordere Zentralwindung bei Pyramidenbahn-Läsionen verschiedener Art. Acta Med Scand (Stockh) 78 [Suppl 46]:1–234
Author information
Authors and Affiliations
Additional information
Supported by the Netherlands Amyotrophic Lateral Sclerosis Foundation
Rights and permissions
About this article
Cite this article
Troost, D., Sillevis Smitt, P.A.E., de Jong, J.M.B.B. et al. Neurofilament and glial alterations in the cerebral cortex in amyotrophic lateral sclerosis. Acta Neuropathol 84, 664–673 (1992). https://doi.org/10.1007/BF00227744
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00227744