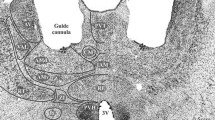
Summary
This study extends an ongoing analysis of the neural mediation of discriminative avoidance learning in rabbits. Electrolytic lesions encompassing anterior and posterior cingulate cortex (area 24 and 29) or ibotenic acid lesions in area 24 only were made prior to avoidance conditioning wherein rabbits learned to step in response to a tone conditional stimulus (CS+) in order to avoid a brief, response-terminated 1.5 mA. foot-shock unconditional stimulus (US). The US was presented 5 s after CS+ onset, in the absence of a prior stepping response. The rabbits also learned to ignore a different tone (CS-) not followed by the US. Multi-unit activity of the caudate and medial dorsal (MD) thalamic nuclei, projection targets of the cingulate cortex, was recorded during learning in all rabbits. Activity was also recorded in area 29 in the rabbits with area 24 lesions. Learning in rabbits with combined lesions was severely impaired and it was moderately retarded after lesions in area 24. MD thalamic and caudate training-induced neuronal discharge increments elicited by the CS+ were enhanced in rabbits with lesions, suggesting a suppressive influence of cingulate cortical projections on this activity. Early-, but not late-developing training-induced unit activity in area 29c/d was absent in rabbits with area 24 lesions, indicating that area 24 is a source of early-developing area 29 plasticity. These results are consistent with hypotheses of a theoretical working model, stating that: a) learning depends on the integrity of two functional systems, a mnemonic recency system comprised by circuitry involving area 24 and the MD nucleus and a mnemonic primacy system comprised by circuitry involving area 29 and the anterior thalamic nuclei; b) corticothalamic information flow in these systems suppresses thalamic CS elicited activity in trained rabbits; c) corticostriatal information flow is involved in avoidance response initiation. An absence of rhythmic theta-like neuronal bursts in area 29b in rabbits with area 24 lesions is attributable to passing fiber damage.
Similar content being viewed by others
References
Barnes CL, Van Hoesen G, Yeterian EH (1980) Widespread projections to the striatum from the limbic mesocortices in the monkey. Soc Neurosci Abstr 100. 12:271
Beckstead RM (1979) Convergent prefrontal and nigral projections to the striatum of the rat. Neurosci Lett 12:59–64
Brogden WJ, Culler FA (1936) A device for motor conditioning of small animals. Science 83:269
Buchwald J, Holstein SB, Weber DS (1973) Multiple unit recording: technique, interpretation, and experimental applications. In: Thompson RF, Patterson MM (eds) Bioelectric recording techniques Part A. Cellular processes and brain potentials. Academic Press, New York, pp 202–242
Bures J, Petran M, Zachar J (1967) Electrophysiological methods in biological research. Academic Press, New York
Carman JB, Cowan MW, Powell TPS (1963) The organization of the corticostriate connections in the rabbit. Brain 86:525–562
Carman JB, Cowan MW, Powell TPS, Webster KEA (1965) A bilateral corticostriate projection. J Neurol Neurosurg Psychiat 28:71–77
Domesick VB (1972) Thalamic relationships of the medial cortex in the rat. Brain Behav Evol 6:457–483
Donovick PJ (1974) A metachromatic stain for neural tissue. Stain Technol 49:49–51
Fox CA, Eichman J (1959) A rapid method for locating intracerebral electrode tracks. Stain Technol 34:39–42
Funahashi S, Bruce CJ, Goldman-Rakic PS (1989) Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophys 61:331–349
Fuster MJ (1985) The prefrontal cortex and temporal integration. In: Peters A, Jones EG (eds) Cerebral cortex, Vol. IV. Plenum Press, New York, pp 151–177
Gabriel M (1991) Functions of anterior and posterior cingulate cortex during avoidance learning in rabbits. In HBM Uylings, Van Eden CG, de Bruin JPC, Feenstra MGP, Corner MA, Eikelboom T, Janssen A (eds.) The prefrontal cortex: its structure function and pathology, Progress in brain research. Elsevier North Holland, Amsterdam, 85:457–473
Gabriel M, Cox A, Perrine CE, Miller JD (1990) Brainstem substrates of conditioning and learning. In: Vertes RP, Klemm WR (eds) Brainstem mechanisms of behavior. John Wiley and Sons, New York, pp 269–314
Gabriel M, Foster K, Orona E, Saltwick SE, Stanton M (1980) Neuronal activity of cingulate cortex, anteroventral thalamus and hippocampal formation in discriminative conditioning: encoding and extraction of the significance of conditional stimuli. In: Sprague J, Epstein AN (eds) Progress in psychobiology and physiological psychology, Vol. 9. Academic Press, New York, pp 126–223
Gabriel M, Kubota Y, Shenker J (1988) Limbic circuit interactions during learning. In: Markowitsch H (eds) Information processing by the brain, Hans Huber Publishers, Toronto, pp 39–63
Gabriel M, Lambert RW, Foster K, Orona E, Sparenborg S, Maiorca RR (1983) Anterior thalamic lesions and neuronal activity in the cingulate and retrosplenial cortices during discriminative avoidance behavior in rabbits. Behav Neurosci 97:675–696
Gabriel M, Orona E (1982) Parallel and serial processes of the prefrontal and cingulate cortical systems during behavioral learning. Brain Res Bull 8:781–785
Gabriel M, Sparenborg S, Kubota Y (1989) Anterior and medial thalamic lesions, discriminative avoidance learning and cingulate cortical neuronal activity in rabbits. Exp Brain Res 76:441–457
Gabriel M, Sparenborg S, Stolar N (1987) Hippocampal control of cingulate cortical and anterior thalamic information processing during learning in rabbits. Exp Brain Res 67:131–152
Girgis M, Shi-Chang W (1981) A new stereotaxic atlas of the rabbit brain. Warren H Green Inc, Saint Louis
Kang E, Kubota Y, Poremba A, Gabriel M (1990) Hippocampal lesions, limbic cortical and thalamic training-induced unit activity, avoidance conditioning, and response to different forms of novelty in rabbits. Soc Neurosci Abstr 113. 18:264
Kemp JM, Powell TPS (1970) The cortico-striate projection in the monkey. Brain Res 93:525–546
Kubota Y, Holmbo A, Poremba A, Kang E, Gabriel M (1990) Cholinergic diagonal band lesions and limbic cortical and thalamic unit activity during learning in rabbits. Soc Neurosci Abstr 113. 17:264
Markowska AL, Olton DS, Murray EA, Gaffan D (1989) A comparative analysis of the role of fornix and cingulate cortex in memory: rats. Exp Brain Res 74:187–201
Mignard M, Bentzinger D, Bender N, Gabriel M (1987) Type I and type II theta-like unit activity in structures of the Papez circuit during differential avoidance conditioning in rabbits. Soc Neurosci Abstr 13:1103
Mishkin M, Appenzeller T (1987) The anatomy of memory. Sci Am 256:80–89
Mogensen GJ (1987) Limbic-motor integration. In: Epstein AN (eds) Progress in psychobiology and physiological psychology. Academic Press, Orlando, pp 117–170
Morrison JH, Molliver ME, Grzanna R, Coyle JT (1981) The intra-cortical trajectory of the coeruleo-cortical projection in the rat: A tangentially organized cortical afferent. Neuroscience 6:139–158
Olton DS, Becker JT, Handelmann GE (1979) Hippocampus, space and memory. Behav Brain Sci 2:313–365
Orona E, Gabriel M (1983) Unit activity of the prefrontal cortex and the mediodorsal thalamic nucleus during acquisition of discriminative avoidance behavior in rabbits. Brain Res 263:295–312
Packard MG, Hirsh R, White NM (1989) Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: evidence for multiple memory systems. J Neurosci 9:1465–1472
Packard MG, White NM (1990) Lesions of the caudate nucleus selectively impair reference memory in the radial maze. Behav Neurol Biol 53:39–50
Passingham RE (1985) Memory of monkeys (Macaca mulatta) with lesions in prefrontal cortex. Behav Neurosci 99:3–21
Peterson SL (1986) Prefrontal cortex neuron activity during a discriminative conditioning paradigm in unanesthetized rats. Int J Neurosci 29:245–254
Pirch JH, Corbus MJ, Rigdon GC (1985) Conditioning-related single-unit activity in the frontal cortex of urethane anesthetized rats. Int J Neurosci 25:263–271
Rescorla RA (1967) Pavlovian conditioning and its proper control procedures. Psychol Rev 74:71–80
Sparenborg S, Gabriel M (1987) Limbic system unit activity and behavior altered during learning by local depletions of norepi≠phrine in rabbits. Soc Neurosci Abstr 184. 14:657
Sparenborg S, Gabriel M (1990) Neuronal encoding of conditional stimulus duration in cingulate cortex of rabbits. Behav Neurosci 104:919–933
Squire LR (1987) Memory and brain. Oxford University Press, New York
Squire LR, Moore RY (1979) Dorsal thalamic lesions in a noted case of human memory dysfunction. Ann Neurol 6:503–506
Stokes KA, Best PJ (1988) Mediodorsal thalamic lesions impair radial maze performance in the rat. Behav Neurosci 102:294–300
Stokes KA, Best PJ (1989) Mediodorsal thalamus lesions in rats impair radial-arm maze performance in a cued environment. Psychobiology 18:63–67
Stolar N, Sparenborg S, Donchin E, Gabriel M (1989) Conditional stimulus probability and activity of hippocampal, cingulate cortical and limbic thalamic neurons during avoidance conditioning in rabbits. Behav Neurosci 103:919–934
Sutherland RJ, Whishaw IQ, Kolb B (1988) Contributions of cingulate cortex to two forms of spatial learning and memory. J Neurosci 8:1863–1872
Thompson RF (1986) The neurobiology of learning and memory. Science 223:941–947
Thompson RL (1959) Effects of lesions in the caudate nuclei and dorsofrontal cortex on conditioned avoidance behavior in cats. J Comp Physiol Psychol 52:650–659
Toan DL, Schultz W (1985) Responses of rat pallidum cells to cortex stimulation and effects of altered dopaminergic activity. Neuroscience 15:683–694
Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT (1987) Retrosplenial amnesia. Brain 110:1631–1646
Vogt BA (1985) The cingulate cortex. In: Jones EG, Peters A (eds) Cerebral cortex. Plenum Press, New York, pp 89–149
Vogt BA, Miller MW (1983) Cortical connections between rat cingulate cortex and visual, motor and postsubicular cortices. J Comp Neurol 216:192–210
Vogt BA, Sikes RW, Swadlow HA, Weyand TG (1986) Rabbit cingulate cortex: cytoarchitecture, physiological border with visual cortex and afferent cortical connections of visual, motor, postsubicular and intracingulate origin. J Comp Neurol 248:74–94
Walker HM, Lev J (1953) Statistical inference. Holt, New York
Wilson CJ, Chang HT, Kitai ST (1982) Origins of postsynaptic potentials evoked in identified rat neostriatal neurons by stimulation in substantia nigra. Exp Brain Res 45:157–167
Winer BJ (1962) Statistical principles in experimental design. McGraw-Hill, New York
Yeterian EH, Van Hoesen GW (1978) Corticostriate projections in the rhesus monkey: the organization of certain cortico-caudate connections. Brain Res 139:43–63
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gabriel, M., Kubota, Y., Sparenborg, S. et al. Effects of cingulate cortical lesions on avoidance learning and training-induced unit activity in rabbits. Exp Brain Res 86, 585–600 (1991). https://doi.org/10.1007/BF00230532
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00230532