Summary
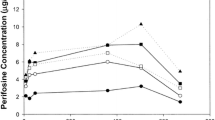
Trimetrexate glucuronate (TMTX) is a methotrexate (MTX) analog that is active against transport-deficient MTX-resistant tumor cells. We performed a phase I study of TMTX administered by daily bolus for 9 consecutive days since this schedule is one of the most active in experimental murine tumor models. The drug was administered in this fashion every 4 weeks for at least two cycles. Fifteen patients with refractory metastatic cancers were studied and all had received prior chemotherapy. The dose-limiting toxicity was a rapidly reversible thrombocytopenia first seen at a daily dose of 4.0 mg/m2 which occurred 7 days after the end of TMTX administration. There was great inter-and intrapatient variability in the platelet nadirs observed in the six patients treated at 4.0 mg/m2. One patient died of massive hemoptysis during a platelet nadir at that dose level. Granulocyte counts never dropped below 1500/mm3. Only one patient had significant non-hematological toxicity: a radiation recall skin toxicity along with a self-limited maculopapular rash. One patient with melanoma and lung metastases treated at 4.0 mg/m2 had a partial response. TMTX plasma levels were measured by HPLC every 3 days prior to daily dosing in patients receiving 4 mg/m2 to determine whether drug accumulation occurred during this prolonged administration schedule. Nadir drug levels varied from less than 0.02 to 0.35 μM and did not seem to increase during the 9-day schedule in individual patients. By comparison with other phase I trials, the hematologic toxicity of TMTX seems to be schedule-dependent, with less drug being tolerated and more severe thrombocytopenia observed with more protracted treatment protocols. A firm phase II starting dose for daily bolus x 9 schedules is difficult to recommend in view of the variable toxicity observed in the patients treated at 4.0 mg/m2 daily, who, in addition, had all been extensively pretreated. A reasonable starting dose might be 3.0 mg/m2 daily with built-in dosage increases or decreases.
Similar content being viewed by others
References
Ackerly CC, Harthshorn J, Tong WP, McCormack JJ (1985) A rapid and sensitive method for determination of trimetrexate from biological fluids. J Liquid Chromatogr 8: 125–134
Balis FM, Lester CM, Poplack DG (1986) Pharmacokinetics of trimetrexate in monkeys. Cancer Res 46: 169–174
Cowan KH, Jolivet J (1984) A methotrexate-resistant human breast cancer cell line with multiple defects, including diminished formation of methotrexate polyglutamates. J Biol Chem 259: 10793–10800
Diddens H, Niethammer D, Jackson RC (1983) Patterns of cross-resistance to the antifolate drugs, trimetrexate, metoprine, homofolate and CB 3717 in human lymphoma and osteosarcoma cells resistant to methotrexate. Cancer Res 43: 5286–5292
Donehower RC, Graham ML, Thompson GE, Dole GB, Ettinger DS (1985) Phase I and pharmacokinetic study of trimetrexate in patients with advanced cancer. Proc Am Soc Clin Oncol 4: 32 (abstract C-118)
Fanucchi M, Fleisher M, Vidal P, Williams L, Bauer T, Cassidy C, Chou T-C, Young C (1985) Phase I and pharmacologic study of trimetrexate. Proc Am Ass Cancer Res 26: 179 (abstract 710)
Goldin A, Mantel N, Greenhouse SW, Venditti JM, Humphreys SR (1954) Factors influencing the specificity of activity of an antileukemic agent (aminopterin). Time of treatment and dosage schedule. Cancer Res 14: 311–314
Ho DHN, Covington, WP, Legha S, Newman RA, Krakoff IH (1986) Clinical pharmacology of trimetrexate. Proc Am Ass Cancer Res 27: 173 (abstract 687)
Jackson RC, Fry DW, Boritzki TJ, Besserer JA, Leopold WR, Sloan BJ, Elslager EF (1984) Biochemical pharmacology of the lipophilic antifolate trimetrexate. Adv Enzyme Regul 22: 187–206
Kamen BA, Eibl B, Cashmore A, Bertino J (1984) Uptake and efficacy of trimetrexate, a non-classical antifolate in methotrexate-resistant leukemia cells in vitro. Biochem Pharmacol. 33: 1697–1699
Legha S, Tenney D, Ho DH, Krakoff IH (1985) Phase I clinical and pharmacology study of trimetrexate. Proc Am Soc Clin Oncol 4: 48 (abstract C-183)
Lin JT, Cashmore AR, Baker M, Dreyer RN, Ernstoff M, Marsh JC, Bertino JR, Whitfield LR, Delap R, Grillo-Lopez AJ (1987) Phase I studies with trimetrexate: clinical pharmacology, analytical methodology and pharmacokinetics. Cancer Res 47: 609–616
Mini E, Moroson BA, Franco CT, Bertino JR (1985) Cytotoxic effects of folate antagonists against methotrexate-resistant human leukemic lymphoblast CCRF-CEM cell lines. Cancer Res 45: 325–330
National Cancer Institute (U.S.) (1983) Clinical brochure of trimetrexate glucuronate (NSC 352122). November
O'Dwyer PJ, Shoemaker DD, Plowman J, Cradock J, Grillo-Lopez AJ, Leyland-Jones B (1985) Trimetrexate: a new antifolate entering clinical trials. Invest. New Drugs 3: 71–75
Ohnoshi T, Ohnuma J, Takahashi I, Scanlon K, Kamen BA, Holland JF (1982) Establishment of methotrexate-resistant human acute lymphoblastic leukemia cells in culture and effects of folate antagonists. Cancer Res 42: 1655–1660
Rosen M, Ohnuma A, Zimet V, Coffey V, Zhang W, Holland JF (1986) Phase I study of trimetrexate glucuronate in a 5-day infusion schedule. Proc Am Ass Cancer Res 27: 172 (abstract 681)
Stewart JA, McCormack JJ, Tong W, Delap RJ, Grillo-Lopez AJ (1985) A phase I study of trimetrexate. Proc Am Ass Cancer Res 26: 159 (abstract 631)
Weiss RB, James WD, Major WB, Porter MB, Allegra CJ, Curt GA (1986) Skin reactions induced by trimetrexate, an analog of methotrexate. Invest New Drugs 4: 159–163
Author information
Authors and Affiliations
Additional information
Supported by the National Cancer Institute of Canada Clinical Trials Group.
Rights and permissions
About this article
Cite this article
Jolivet, J., Landry, L., Pinard, MF. et al. A phase I study of trimetrexate, an analog of methotrexate, administered monthly in the form of nine consecutive daily bolus injections. Cancer Chemother. Pharmacol. 20, 169–172 (1987). https://doi.org/10.1007/BF00253973
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00253973