Summary
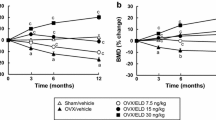
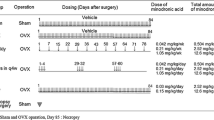
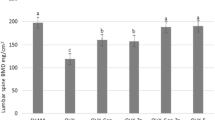
The present study investigated the prophylactic effects of vitamin D metabolites and vitamin D metabolite combinations on static and dynamic, tetracycline-based, histomorphometric parameters in the axial skeleton of ovariectomized rats. Forty-three Fischer-344 rats (10 weeks old, 130 g each body weight, BW) were either bilaterally ovariectomized (OVX) or sham-operated (SHAM). The rats were allocated into the following groups: SHAM; OVX; OVX+7.5 ng 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3]/rat/day; OVX +15 ng 1α,24R,25-trihydroxyvitamin D3 [1,24,25-(OH)3D3]/rat/day; OVX+75 ng 24R,25-dihydroxyvitamin D3 [24,25(OH)2D3]/rat/day; OVX+7.5 ng 1,25(OH)2D3/rat/ day+15 ng 1,24,25(OH)3D3/rat/day; OVX+7.5 ng 1,25(OH)2D3/rat/day+75 ng 24,25(OH)2D3/rat/day. The vitamin D metabolites were fed orally starting 4 weeks after surgery. Urine and blood samples were collected 12 and 16 weeks postovariectomy, respectively. Sixteen weeks after surgery, all rats were sacrificed, and the first lumbar vertebrae were processed undecalcified for histomorphometric analysis. Ovariectomy induced a highly significant reduction (P<0.001) of cancellous bone mass in the secondary spongiosa of the lumbar vertebral body. The bone loss in OVX rats was accompanied by a distinct elevation of all histomorphometric parameters of bone formation and resorption. 1,25(OH)2D3 and both vitamin D metabolite combinations significantly raised serum calcium levels and prevented the bone loss by inhibiting the increased bone resorption in OVX rats. In the applied dosage, 1,24,25(OH)3D3 and 24,25(OH)2D3 alone were ineffective in preserving the cancellous bone of the lumbar vertebra in OVX rats. We conclude that the oral prophylactic application of low doses of active vitamin D metabolites can effectively prevent the osteopenia induced by ovariectomy in the axial skeleton of the rat.
Similar content being viewed by others
References
Reichel H, Koeffler HP, Norman AW (1989) The role of the vitamin D endocrine system in health and disease. N Engl J Med 320: 980–991
Miller SC, Halloran BP, DeLuca HF, Yamada S, Takayama H, Jee WSS (1981) Studies on the role of 24-hydroxylation of vitamin D in the mineralization of cartilage and bone of vitamin D-deficient rats. Calcif Tissue Int 33: 489–497
Brommage R, Jarnagin K, DeLuca HF, Yamada S, Takayama H (1983) 1-But not 24-hydroxylation of vitamin D is required for skeletal mineralization in rats. Am J Physiol 244: E298-E304
Parfitt AM, Mathews CHE, Brommage R, Jarnagin K, DeLuca HF (1984) Calcitriol but no other metabolite of vitamin D is essential for normal bone growth and development in the rat. J Clin Invest 73: 576–586
Wientroub S, Reddi AH (1982) Vitamin D metabolites and endochondral bone development. In: Silberman M, Slavkin HC (eds) Current advances in skeletogenesis: development, biomineralization, metabolic bone disease. Excerpta Media, Amsterdam, pp 211–217
Atkin I, Pita JC, Ornoy A, Agundez A, Castiglione G, Howell DS (1985) Effects of vitamin D metabolites on healing of low phosphate, vitamin D-deficient induced rickets in rats. Bone 6: 113–123
Tam CS, Heersche JNM, Jones G, Murray TM, Rasmussen H (1986) The effect of vitamin D on bone in vivo. Endocrinology 118: 2217–2224
Lidor C, Atkin I, Ornoy A, Dekel S, Edelstein S (1987) Healing of rachitic lesions in chicks by 24R,25-dihydroxycholecalciferol administered locally into bone. J Bone Miner Res 2: 91–98
Hinek A, Poole AR (1988) The influence of vitamin D metabolites on the calcification of cartilage matrix and the C-propeptide of type II collagen (chondrocalcin). J Bone Miner Res 3: 421–429
Takigawa M, Enomoto M, Shirai E, Nishii Y, Suzuki F (1988) Differential effects of 1α,25-dihydroxycholecalciferol and 24R,25-dihydroxycholecalciferol on the proliferation and the differentiated phenotype of rabbit costal chondrocytes in culture. Endocrinology 122: 831–839
Rambeck WA, Zucker H (1985) Synergistic effects of 1,25(OH)2D3 and 24,25(OH)2D3 on duodenal CaBP in rachitic chicks and on eggshell weight in Japanese quails. Biochem Biophys Res Commun 126: 799–804
Rambeck WA, Weiser H, Meier W, Zucker H (1987) Synergistic effects of 1,25(OH)2D3 and 1,24,25(OH)3D3 in rachitic chicken and rats. In: Kuhlencordt F, Dietsch P, Keck E, Kruse H-P (eds) Generalized bone diseases. Springer, Berlin, New York, pp 169–173
Rambeck WA, Weiser H, Meier W, Zucker H (1988) Synergistic effects of vitamin D metabolites. Ann Nutr Metab 32: 108–111
Christiansen C, Christensen MS, McNair P, Hagen C, Stocklund K-E, Transbøl I (1980) Prevention of early postmenopausal bone loss: controlled 2-year study in 315 normal females. Eur J Clin Invest 10: 273–279
Christiansen C, Christensen MS, Rødbro P, Hagen C, Transbøl I (1981) Effect of 1,25-dihydroxyvitamin D3 in itself or combined with hormone treatment in preventing postmenopausal osteoporosis. Eur J Clin Invest 11: 305–309
Riis BJ, Thomsen K, Christiansen C (1986) Does 24R,25(OH)2-vitamin D3 prevent postmenopausal bone loss? Calcif Tissue Int 39: 128–132
Aloia JF, Vaswani A, Yeh J, Ellis K, Cohn SH (1987) Treatment of postmenopausal osteoporosis with calcitriol. In: Christiansen C, Johansen JS, Riis BJ (eds) Osteoporosis 1987. Osteopress ApS, Copenhagen, pp 850–852
Caniggia A, Nuti R, Loré F, Martini G, Righi GA, Turchetti V (1988) Long-term calcitriol treatment in postmenopausal osteoporosis: follow-up of two hundred patients. In: Norman AW, Schaefer K, Grigoleit H-G, von Herrath D (eds) Vitamin D. Molecular, cellular and clinical endocrinology. Walter de Gruyter, Berlin, New York, pp 807–816
Gallagher JC, Goldgar D, O'Neill J (1988) Calcitriol therapy in the management of osteoporosis. In: Norman AW, Schaefer K, Grigoleit H-G, von Herrath D (eds) Vitamin D. Molecular, cellular and clinical endocrinology. Walter de Gruyter, Berlin, New York, pp 836–837
Hodgkinson A, Aaron JE, Horsman A, McLachlan MSF, Nordin BEC (1978) Effect of oophorectomy and calcium deprivation on bone mass in the rat. Clin Sci Mol Med 54: 439–446
Wronski TJ, Walsh CC, Ignaszewski LA (1986) Histologic evidence for osteopenia and increased bone turnover in ovariectomized rats. Bone 7: 119–123
Wronski TJ, Dann LM, Horner SL (1989) Time course of vertebral osteopenia in ovariectomized rats. Bone 10: 295–301
Baron R, Tross R, Vignery A (1984) Evidence of sequential remodeling in rat trabecular bone: morphology, dynamic histomorphometry, and changes during skeletal maturation. Anat Rec 208: 137–145
Lindgren JU, Lindholm TS (1979) Effect of 1-alpha-hydroxyvitamin D3 on osteoporosis in rats induced by oophorectomy. Calcif Tissue Int 27: 161–164
Lindgren JU, DeLuca HF (1982) Role of parathyroid hormone and 1,25-dihydroxyvitamin D3 in the development of osteopenia in oophorectomized rats. Calcif Tissue Int 34: 510–514
Izawa Y, Sagara K, Kadota T, Makita T (1985) Comparison of therapeutic usefulness of vitamin D3 analogs on osteoporosis caused by ovariectomy in rats. Acta Vitaminol Enzymol 7: 173–182
Matsumoto T, Ezawa I, Morita K, Kawanobe Y, Ogata E (1985) Effect of vitamin D metabolites on bone metabolism in a rat model of postmenopausal osteoporosis. J Nutr Sci Vitaminol 31 (suppl): S61-S65
Faugere M-C, Okamoto S, DeLuca HF, Malluche HH (1986) Calcitriol corrects bone loss induced by oophorectomy in rats. Am J Physiol 250: E35-E38
Erben RG, Kohn B, Rambeck WA, Zucker H (1990) Histomorphometric analysis of the rat proximal tibial metaphysis by “linear scanning.” Scanning Microsc 4: 625–640
Kohn B, Erben RG, Rambeck WA, Zucker H (in press) Osteopenia caused by ovariectomy in young female rats and prophylactic effects of 1,25-dihydroxyvitamin D3. J Vet Med
Stegemann H (1958) Mikrobestimmung von Hydroxyprolin mit Chloramin-T und p-Dimethylaminobenzaldehyd (Microassay of hydroxyproline with chloramine T and p-dimethyl aminobenzaldehyde). Z Physiol Chem 311: 41–45
Baron R, Vignery A, Neff L, Silverglate A, Santa Maria A (1983) Processing of undecalcified bone specimens for bone histomorphometry. In: Recker RR (ed) Bone histomorphometry: techniques and interpretation. CRC Press, Boca Raton, Florida, pp 13–35
Schenk RK, Olah AJ, Herrmann W (1984) Preparation of calcified tissues for light microscopy. In: Dickson GR (ed) Methods of calcified tissue preparation. Elsevier Science Publishers B.V., Amsterdam, New York, pp 1–56
Kimmel DB, Jee WSS (1980) A quantitative histologic analysis of the growing long bone metaphysis. Calcif Tissue Int 32: 113–122
Parfitt AM (1983) The physiologic and clinical significance of bone histomorphometric data. In: Recker RR (ed) Bone histomorphometry: techniques and interpretation. CRC Press, Boca Raton, Florida, pp 143–223
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche HH, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. J Bone Miner Res 2: 595–610
Kimmel DB, Jee WSS (1983) Measurements of area, perimeter, and distance: details of data collection in bone histomorphometry. In: Recker RR (ed) Bone histomorphometry: techniques and interpretation. CRC Press, Boca Raton, Florida, pp 89–108
Wronski TJ, Cintrón M, Dann LM (1988) Temporal relationship between bone loss and increased bone turnover in ovariectomized rats. Calcif Tissue Int 43: 179–183
Turner RT, Wakley GK, Hannon KS, Bell NH (1988) Tamoxifen inhibits osteoclast-mediated resorption of trabecular bone in ovarian hormone-deficient rats. Endocrinology 122: 1146–1150
Hui SL, Slemenda CW, Johnson CC, Appledorn CR (1987) Effects of age and menopause on vertebral bone density. Bone Miner 2: 141–146
Heaney RP, Recker RR, Saville PD (1978) Menopausal changes in bone remodeling. J Lab Clin Med 92: 964–970
Pødenphant J, Johansen JS, Thomsen K, Riis BJ, Leth A, Christiansen C (1987) Bone turnover in spinal osteoporosis. J Bone Miner Res 2: 497–503
Parfitt AM, Mathews CHE, Villanueva AR, Kleerekoper M, Frame B, Rao DS (1983) Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis. Implications for the microanatomic and cellular mechanisms of bone loss. J Clin Invest 72: 1396–1409
Parfitt AM (1984) Age-related structural changes in trabecular and cortical bone: cellular mechanisms and biomechanical consequences. Calcif Tissue Int 36: S123-S128
Recker RR, Kimmel DB, Parfitt AM, Davies KM, Keshawarz N, Hinders S (1988) Static and tetracycline-based bone histomorphometric data from 34 normal postmenopausal females. J Bone Miner Res 3: 133–144
Fallon MD, Whyte MP, Craig RB Jr, Teitelbaum SL (1983) Mast-cell proliferation in postmenopausal osteoporosis. Calcif Tissue Int 35: 29–31
Peck WA, Woods WL (1988) The cells of bone. In: Riggs BL, Melton LJ III (eds) Osteoporosis: etiology, diagnosis, and management. Raven Press, New York, pp 1–44
Frost HM (1976) Some concepts crucial to the effective study of bone turnover and bone balance in human skeletal disease and in experimental models of skeletal physiology and pathophysiology. In: Jaworski ZFG (ed) Proc 1st Workshop on Bone Morphometry. University of Ottawa Press, Ottawa, pp 219–223
Baron R, Vignery A, Horowitz M (1984) Lymphocytes, macrophages and the regulation of bone remodeling. In: Peck WA (ed) Bone and mineral research. Annual 2. Elsevier Science Publishers B.V., Amsterdam, New York, pp 175–243
Gallagher JA, Beneton M, Harvey L, Lawson DEM (1986) Response of rachitic rat bones to 1,25-dihydroxyvitamin D3:biphasic effects on mineralization and lack of effect on bone resorption. Endocrinology 119: 1603–1609
Hock JM, Gunness-Hey M, Poser J, Olson H, Bell NH, Raisz LG (1986) Stimulation of undermineralized matrix formation by 1,25-dihydroxyvitamin D3 in long bones of rats. Calcif Tissue Int 38: 79–86
Wronski TJ, Halloran BP, Bikle DD, Globus RK, Morey-Holton ER (1986) Chronic administration of 1,25-dihydroxyvitamin D3: increased bone but impaired mineralization. Endocrinology 119: 2580–2585
Gunness-Hey M, Gera I, Fonseca J, Raisz LG, Hock JM (1988) 1,25-dihydroxyvitamin D3 alone or in combination with parathyroid hormone does not increase bone mass in young rats. Calcif Tissue Int 43: 284–288
Frost HM (1981) Mechanical microdamage, bone remodeling, and osteoporosis: a review. In: DeLuca HF, Frost HM, Jee WSS, Johnston CC, Partitt AM (eds) Osteoporosis: recent advances in pathogenesis and treatment. University Park Press, Baltimore, pp 185–190
Suda T, Miyaura C, Abe E, Kuroki T (1986) Modulation of cell differentiation, immune responses and tumor promotion by vitamin D compounds. In: Peck WA (ed) Bone and mineral research/4. Elsevier Science Publishers B.V., Amsterdam, New York, pp 1–48
Martin TJ, Raisz LG, Rodan G (1987) Calcium regulation and bone metabolism. In: Martin TJ, Raisz LG (eds) Clinical endocrinology of calcium metabolism. Marcel Dekker, New York, Basel, pp 1–52
Boivin G, Mesguich P, Pike JW, Bouillon R, Meunier PJ, Haussler MR, Dubois PM, Morel G (1987) Ultrastructural immunocytochemical localization of endogenous 1,25-dihydroxyvitamin D3 and its receptors in osteoblasts and osteocytes from neonatal mouse and rat calvaria. Bone Miner 3: 125–136
Boyce RW, Weisbrode SE (1983) Effect of dietary calcium on the response of bone to 1,25(OH)2D3. Lab Invest 48: 683–689
Okumura H, Yamamuro T, Kasai R, Hayashi T, Tada K, Nishii Y (1988) Effect of 1α-hydroxyvitamin D3 on osteoporosis induced by immobilization combined with ovariectomy in rats. Bone 8: 351–355
Gallagher JC, Jerpbak CM, Jee WSS, Johnson HA, DeLuca HF, Riggs BL (1982) 1,25-dihydroxyvitamin D3: short-and long-term effects on bone and calcium metabolism in patients with postmenopausal osteoporosis. Proc Natl Acad Sci USA 79: 3325–3329
Need AG, Horowitz M, Philcox JC, Nordin BEC (1985) 1,25-dihydroxycalciferol and calcium therapy in osteoporosis with calcium malabsorption. Miner Electrolyte Metab 11: 35–40
Boyce RW, Weisbrode SE (1985) Histogenesis of hyperosteoidosis in 1,25(OH)2D3-treated rats fed high levels of dietary calcium. Bone 6: 105–112
Au WYW (1984) Inhibition by 1,25-dihydroxycholecalciferol of hormonal secretion of rat parathyroid gland in organ culture. Calcif Tissue Int 36: 384–391
Naveh-Many T, Friedlaender MM, Mayer H, Silver J (1989) Calcium regulates parathyroid hormone messenger ribonucleic acid (mRNA), but not calcitonin mRNA in vivo in the rat. Dominant role of 1,25-dihydroxyvitamin D. Endocrinology 125: 275–280
Izawa Y, Makita T, Koyama T, Ohnuma N, Ishimoto S, Inoue T, Orimo H (1982) Protecting effect of active vitamin D analogues on the development of osteoporosis in ovariectomized rats. In: Norman AW, Schaefer K, Grigoleit H-G, von Herrath D (eds) Vitamin D. Chemical, biochemical and clinical endocrinology of calcium metabolism. Walter de Gruyter, Berlin, New York, pp 933–935
Gennari C, Francini G, Civitelli R, Bernini M, Nardi P, Maioli E (1982) 1,25-dihydroxycholecalciferol treatment of postmenopausal osteoporosis. In: Menczel J, Robin GC, Makin M, Steinberg R (eds) Osteoporosis. John Wiley & Sons, Chichester New York, pp 364–372
Buchanan JR, Cauffman SW, Greer RB III (1984) Relation of calcium regulating hormones to the pathogenesis of postmenopausal osteoporosis. In: Christiansen C, Arnaud CD, Nordin BEC, Parfitt AM, Peck WA, Riggs BL (eds) Osteoporosis. Glostrup Hospital, Copenhagen, pp 275–280
Gallagher JC, Recker RR (1985) A comparison of the effects of calcitriol or calcium supplements on bone in postmenopausal osteoporosis. In: Norman AW, Schaefer K, Grigoleit H-G, von Herrath D (eds) Vitamin D. Chemical, biochemical and clinical update. Water de Gruyter, Berlin, New York, pp 971–975
Mundy GR, Roodman GD (1987) Osteoclast ontogeny and function. In: Peck WA (ed) Bone and mineral research/5. Elsevier Science Publishers B.V., Amsterdam, New York, pp 209–279
Marie PJ, Travers R (1983) Continuous infusion of 1,25-dihydroxyvitamin D3 stimulates bone turnover in the normal young mouse. Calcif Tissue Int 35: 418–425
Malluche HH, Matthews C, Faugere M-C, Fanti P, Endres DB, Friedler RM (1986) 1,25-dihydroxyvitamin D maintains bone cell activity, and parathyroid hormone modulates bone cell number in dogs. Endocrinology 119: 1298–1304
Orimo H, Fujita T, Yoshikawa M (1972) Increased sensitivity of bone to parathyroid hormone in ovariectomized rats. Endocrinology 90: 760–763
Heaney RP (1981) Unified concept of the pathogenesis of osteoporosis: updated. In: DeLuca HF, Frost HM, Jee WSS, Johnston CC, Parfitt AM (eds) Osteoporosis: recent advances in pathogenesis and treatment. University Park Press, Baltimore, pp 369–372
Lindgren JU, DeLuca HF (1983) Oral 1,25(OH)2D3: an effective prophylactic treatment for glucocorticoid osteopenia in rats. Calcif Tissue Int 35: 107–110
Goff JP, Reinhardt TA, Beckman MJ, Horst RL (1990) Contrasting effects of exogenous 1,25-dihydroxyvitamin D [1,25-(OH)2D] versus endogenous 1,25-(OH)2D, induced by dietary calcium restriction, on vitamin D receptors. Endocrinology 126: 1031–1035
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Erben, R.G., Weiser, H., Sinowatz, F. et al. Vitamin D metabolites prevent vertebral osteopenia in ovariectomized rats. Calcif Tissue Int 50, 228–236 (1992). https://doi.org/10.1007/BF00296287
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00296287