Abstract
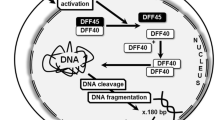
The occurrence and spatial distribution of intracellular DNA fragmentation was investigated by in situ 3′ end labelling of DNA breaks in K562 cells treated in such a way to cause either apoptotic or necrotic cell death. The localisation of DNA breaks was examined by confocal laser microscopy and compared with the electron-microscopic appearance of the cells. In addition, the number of cells with fragmented DNA was counted and compared with the number of dead cells, as determined by the nigrosin dye exclusion test. Apoptosis was induced by cultivation of the cells in the presence of actinomycin D. Cells undergoing apoptosis were characterised by massive intracellular DNA fragmentation that was highly ordered into successive steps. Cells in early stages of the apoptotic process had DNA breaks diffusely distributed in the entire nucleus, except the nucleolus, with crescent-like accumulations beyond the nuclear membrane. In the more advanced stages, the nucleus was transformed into many round bodies with intense labelling. Intracellular accumulations of fragmented DNA corresponded exactly to electron-dense chromatin seen in the electron microscope, whereas diffuse DNA breaks had no morphological correlate at the ultrastructural level. In necrosis induced by ionomycin, NaN3, or rapid freezing combined with thawing, no DNA fragmentation occurred at the onset of cell death, but appeared 24 h later. This fragmentation was not characterised by a unique morphology, but represented the breakdown of the chromatin in the configuration remaining after cell death. Therefore, apoptosis is characterised by DNA fragmentation that proceeds in a regular orderly sequence at the beginning of cell death, and can be detected by in situ 3′end labelling of DNA breaks.
Similar content being viewed by others
References
Afanas'ev VN, Korol' BA, Mantsygin YA, Nelipovich PA, Pechatnikov VA, Umansky SR (1986) Flow cytometry and biochemical analysis of DNA degradation characteristic of two types of cell death. FEBS Lett 194:347–350
Albritton NL, Verret CR, Wolley RC, Eisen HN (1988) Calcium ion concentrations and DNA fragmentation in target cell destruction by murine cloned cytotoxic T lymphocytes. J Exp Med 167:514–527
Arends MJ, Wyllie AH (1991) Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol 32:223–254
Arends MJ, Morris RG, Wyllie AH (1990) Apoptosis: the role of the endonuclease. Am J Pathol 136:593–608
Bowen ID, Bowen SM (1990) Programmed cell death in tumours and tissues, 1st edn. Chapman and Hall, Cambridge
Butyan R (1991) Genetic response of prostata cells to androgen deprivation. In: Tomei LD, Cope FO (eds) Apoptosis: the molecular basis of cell death. Cold Spring Harbor Labaratory, Cold Spring Harbor, NY, pp 157–173
Collins RJ, Harmon BV, Gobe GC, Kerr JFR (1992) Internucleosomal DNA cleavage should not be the sole criterion for identifying apoptosis. Int J Radiat Biol 61:451–453
Cotter TG, Lennon SV, Glynn JG, Martin SJ (1990) Cell death via apoptosis and its relationship to growth, development and differentiation of both tumour and normal cells. Anticancer Res 10:1153–1160
Cotter TG, Glynn JM, Echeverri F, Green DR (1992) The induction of apoptosis by chemotherapeutic agents occurs in all phases of the cell cycle. Anticancer Res 12:773–780
Gavrieli Y, Sherman Y, Ben-Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119:493–501
Gazit B, Cedar H (1982) Active genes are sensitive to deoxyribonuclease I during metaphase. Science 217:648–650
Inouye M, Tamaru M, Kameyama Y (1992) Effects of cycloheximide and actinomycin D on radiation-induced apoptotic cell death in the developing mouse cerebellum. Int J Radiat Biol 61:669–674
Kerr JFR (1965) A histochemical study of hypertrophy and ischaemic injury of rat liver with special reference to changes in lysosomes. J Pathol Bacteriol 90:419–435
Kerr JFR, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Kressel M, Groscurth P (1993) Detection of apoptotic cell death by in situ labeling of DNA double strand breaks. Ann Anat [Suppl] 175:165
Lockwood TD (1988) Distinction between major chloroquine-inhibitable and adrenergic-responsive pathways of protein degradation and their relation to tissue ATP content in the Langendorff isolated perfused rat heart. Biochem J 251:341–346
Maddox PH, Jenkins D (1987) 3-Aminotriethoxysilane: a new advance in section adhesion. J Clin Pathol 40:1256–1260
Martin SJ, Bonham AM, Cotter TG (1990) The involvement of RNA and protein synthesis in programmed cell death in human leukaemia HL-60 cells. Biochem Soc Trans 18:634–636
Ojcius DM, Zychlinsky A, Zheng LM, Ding-E Young J (1991) Ionophore-induced apoptosis: role of DNA fragmentation and calcium fluxes. Exp Cell Res 197:43–49
Pipan N, Sterle M (1979) Cytochemical analysis of organelle degradation in phagosomes and apoptotic cells of the mucoid epithelium of mice. Histochemistry 59:225–232
Schmid SL, Carter LL (1990) ATP is required for receptor-mediated endocytosis in intact cells. J Cell Biol 111:2307–2318
Wotring LL, Passiatore JE, Roti Roti JL, Hudson JL, Townsend LB (1985) Effects of the tricyclic nucleoside 6-amino-4-methyl-8-(β-D-ribofuranosyl)-pyrrolo[4, 3, 2-de]pyrim ido[4, 5-c]pyridazine on the viability and cell cycle distribution of L1210 cells in vitro. Cancer Research 45:6355–6361
Wyllie AH, Morris RG, Smith AL, Dunlop D (1984) Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependance on macromolecular synthesis. J Pathol 142:67–77
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kressel, M., Groscurth, P. Distinction of apoptotic and necrotic cell death by in situ labelling of fragmented DNA. Cell Tissue Res 278, 549–556 (1994). https://doi.org/10.1007/BF00331373
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00331373